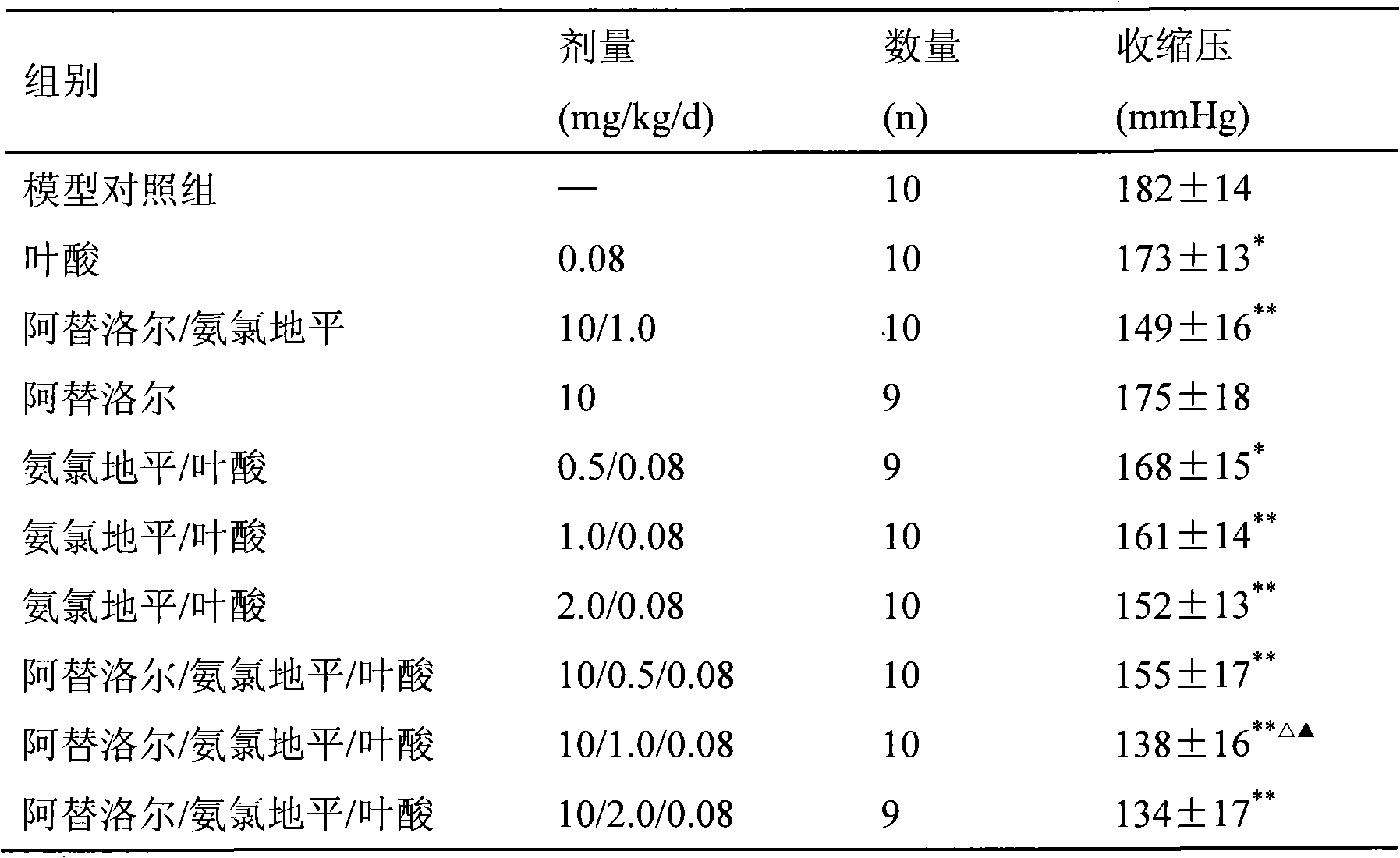
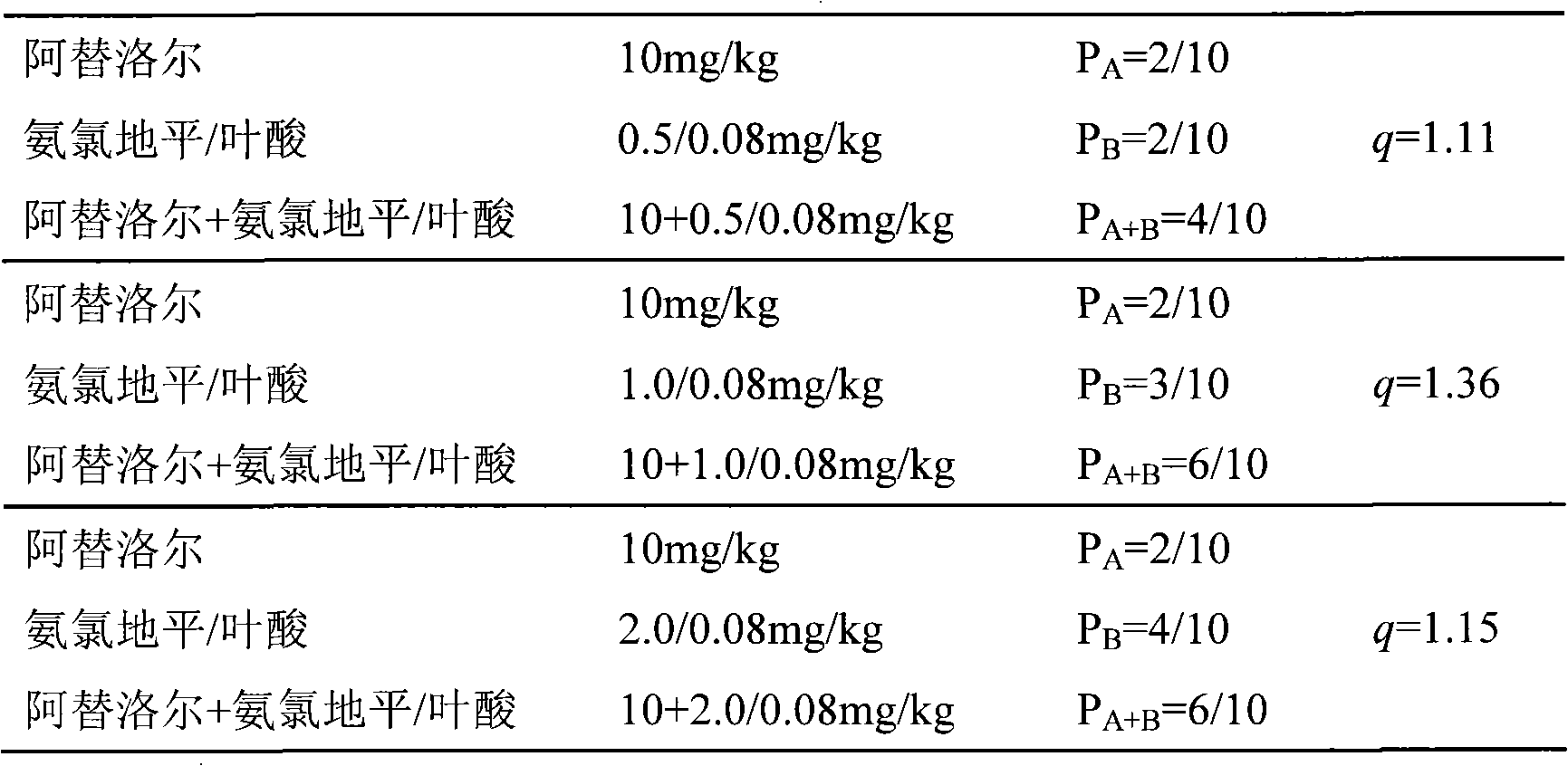
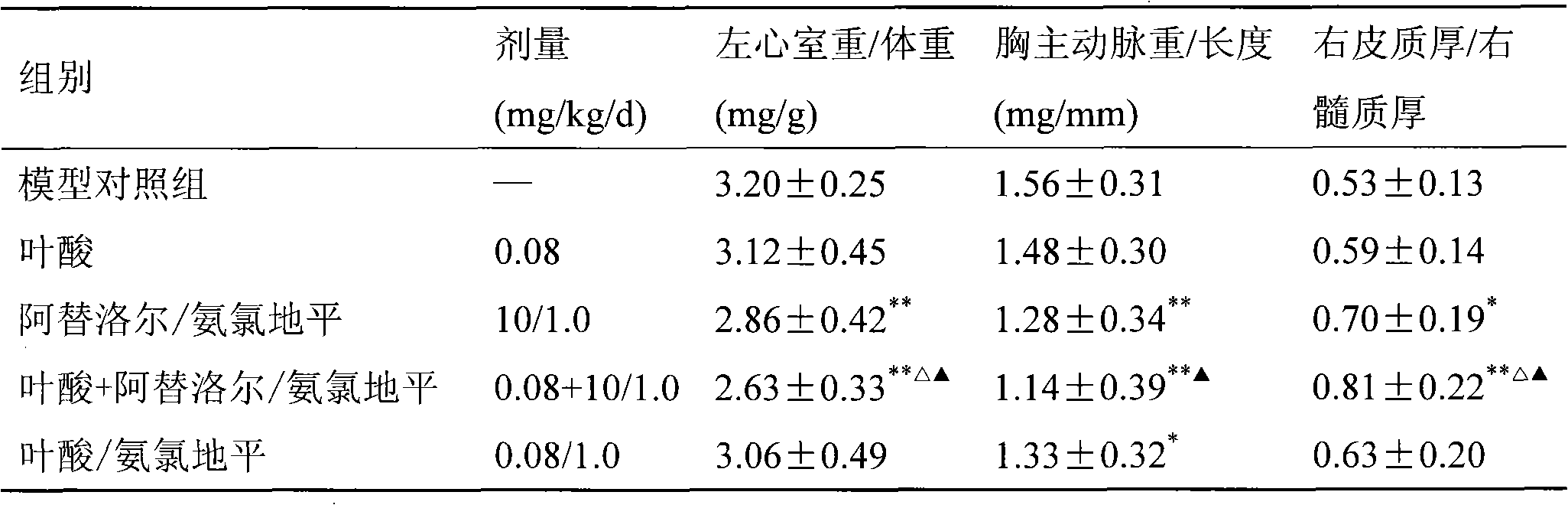
Pharmaceutical composition of atenolol/amlodipine/folacin compound and uses thereof
A technology of amlodipine and levamlodipine, which is applied in the field of pharmacy and can solve the problems of reduced cardiac output and coronary flow, coronary blood flow, bleeding and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of Atenolol 20mg / Amlodipine Besylate 2.0mg / Folic Acid 0.4mg Tablets
[0037] prescription
[0038] Atenolol 20g
[0039] Amlodipine Besylate 2.0g
[0040] Folic acid 0.4g
[0041] Starch 45g
[0042] Microcrystalline Cellulose 45g
[0043] Sodium carboxymethylcellulose (CMS.Na) 5.0g
[0044] 5% povidone k-30 (solvent is absolute ethanol) 1.0g
[0045] Magnesium Stearate Appropriate amount
[0046]
[0047] Made into 1000 pieces
[0048] Preparation Process:
[0049] (1) Take the prescribed amount of atenolol, amlodipine besylate and folic acid and pass through a 100-mesh sieve and then mix them uniformly by the method of increasing in equal amounts for subsequent use;
[0050] (2) Other auxiliary materials are passed through a 100-mesh sieve and dried at 75°C for 2 hours;
[0051] (3) Mix the starch, microcrystalline cellulose, and CMS.Na according to the...
Embodiment 2
[0055] Example 2 Preparation of Atenolol 25mg / Amlodipine Maleate 2.5mg / Leucovorin 0.8mg Capsules
[0056] prescription
[0057] Atenolol 25g
[0058] Amlodipine Maleate 2.5g
[0059] Calcium folinate (as folinic acid) 0.8g
[0060] Lactose 30g
[0061] Microcrystalline Cellulose 15g
[0062] Starch 20g
[0063] Carboxymethyl starch sodium 5.0g
[0064] Magnesium Stearate Appropriate amount
[0065]
[0066] Make 1000 capsules
[0067] Preparation Process:
[0068] According to the prescription ratio, take lactose, microcrystalline cellulose, starch, and carboxymethyl starch sodium and dry them at about 100°C for about 2 hours, pass through a 100-mesh sieve; pass the raw material through a 100-mesh sieve, and increase the amount of the above-mentioned excipients in equal increments Mix well and fill the capsules.
[0069] 1 time a day, 1-2 capsules each time.
Embodiment 3
[0070] Example 3 Preparation of Atenolol 50mg / Amlodipine Besylate 5mg / / Folic Acid 0.8mg / Leucovorin 0.8mg Sustained Release Tablets
[0071] prescription
[0072] Atenolol 50g
[0073] Amlodipine Besylate 5g
[0074] Folic acid 0.8g
[0075] Calcium folinate 0.8g
[0076] Citric acid 10g
[0077] Hypromellose (K4M) 160g
[0078] Lactose 180g
[0079] Magnesium Stearate Appropriate amount
[0080]
[0081] Made into 1000 pieces
[0082] Preparation Process:
[0083] According to the proportion of the prescription, the raw material is mixed with hypromellose, citric acid is dissolved in ethanol as a wetting agent to make a soft material, granulated, dried, sized, mixed with magnesium stearate, pressed Slice and serve.
[0084] 1 time every other day, 1 tablet each time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com