Levamlodipine besylate tablets
A technology of levamlodipine besylate and levo-amlodipine besylate, which is applied in the field of levamlodipine besylate tablets, can solve the problems of complex process, increase of impurities, and decrease of hygroscopicity, and achieve low impurity content and complete dissolution , the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0018] Embodiment 1~3 Levoamlodipine besylate tablet
[0019] Example
[0020] Preparation process: Mix the prescribed amount of levamlodipine besylate and pregelatinized starch, then add other ingredients, mix evenly, and press into tablets to obtain the product.
experiment example 1
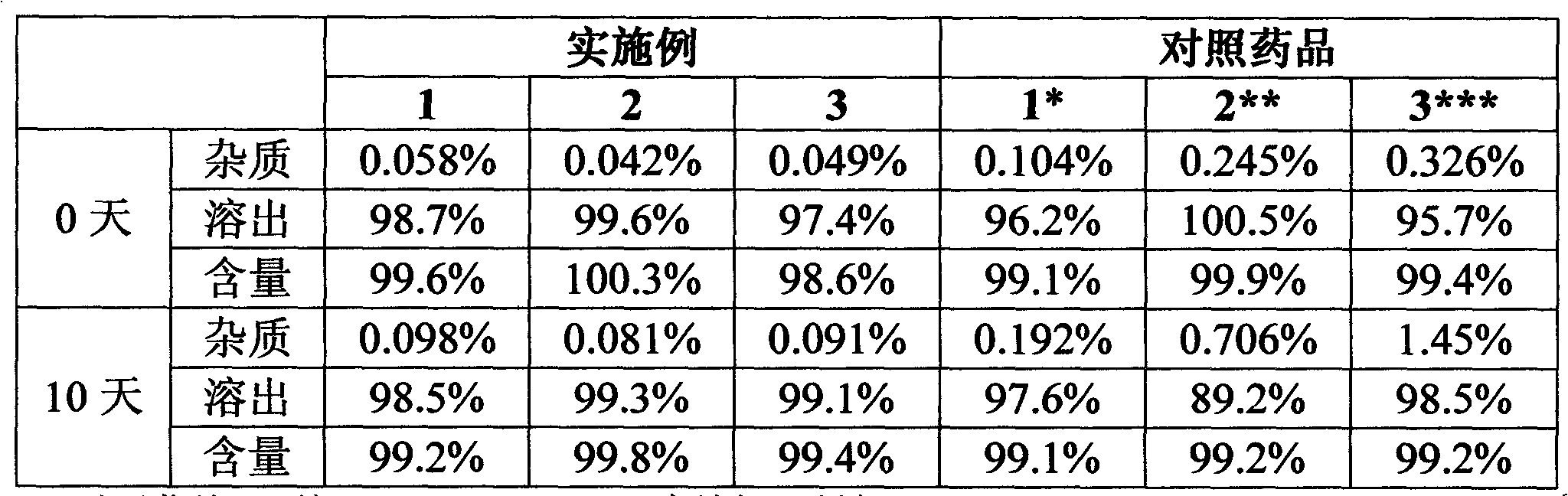
[0021] Experimental Example 1 Stability study under high temperature conditions (60°C, without packaging)
[0022] The levamlodipine besylate sheet that embodiment 1~3 makes is detected by national standard WS1-(X-020)-2002Z, and compares with reference drug 1-3, and the result is as follows:
[0023]
[0024] *Comparative sample 1: prepared according to Example 1 of ZL201010134110.9 (the same below).
[0025] **Comparative drug 2: prepared according to Example 1 of CN200910186862.7 (the same below).
[0026] ***Comparative drug 3: prepared according to Example 1 of ZL200910147904.6 (the same below).
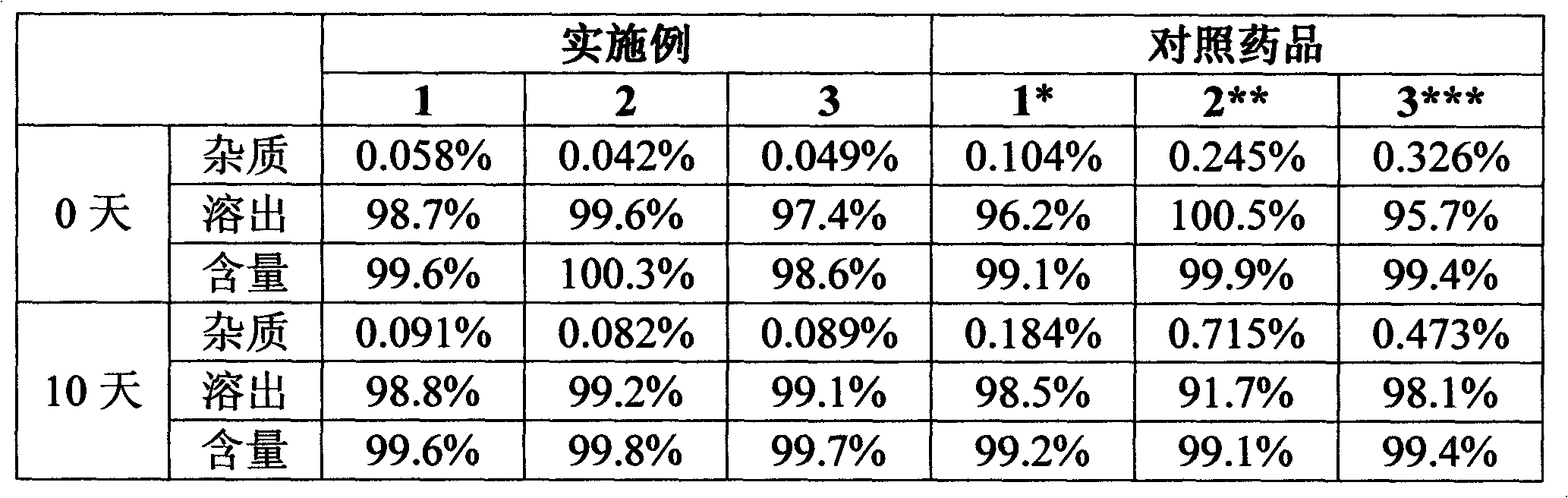
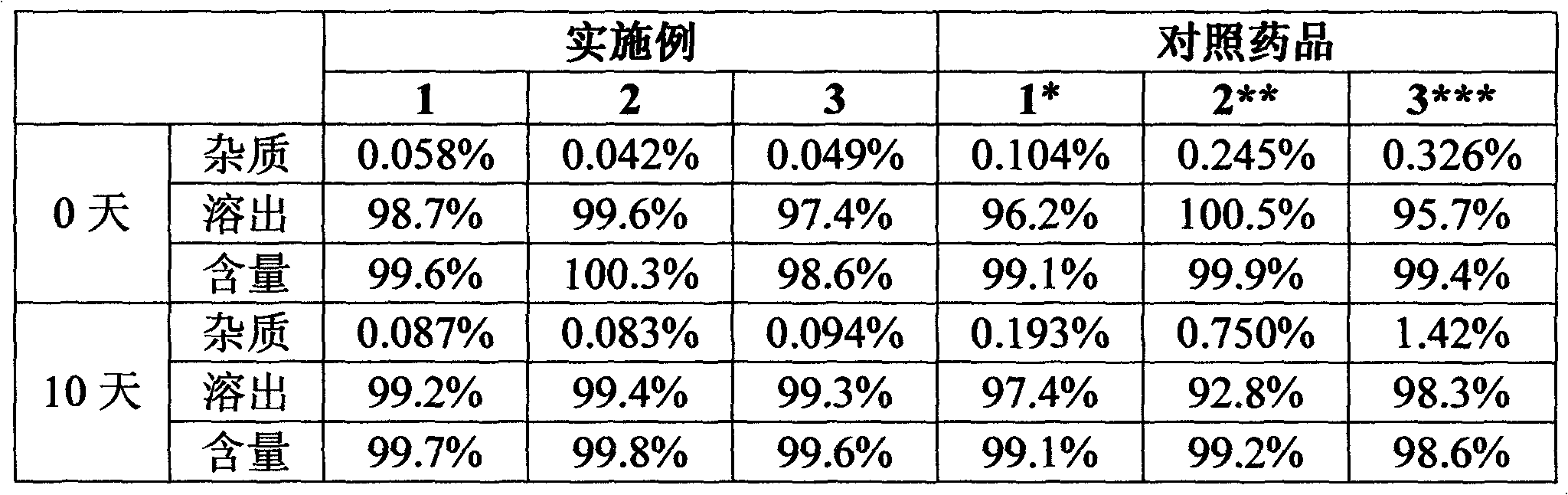
experiment example 2
[0027] Experimental Example 2 Stability study under high humidity conditions (25°C, RH92.5%, no packaging)
[0028] The levamlodipine besylate sheet that embodiment 1~3 makes is detected by national standard WS1-(X-020)-2002Z, and compares with reference drug 1-3, and the result is as follows:
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com