Medicine application preparation for treating hypertension
A technology for high blood pressure and drugs, which is applied in the field of drug application preparations containing levamlodipine and angiotensin II receptor blockers, can solve the problems of no technical solutions for sustained release agents, and achieve benefits for high blood pressure Protection of target organ structure and function, improvement of remission, and reduction of impaired glucose tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
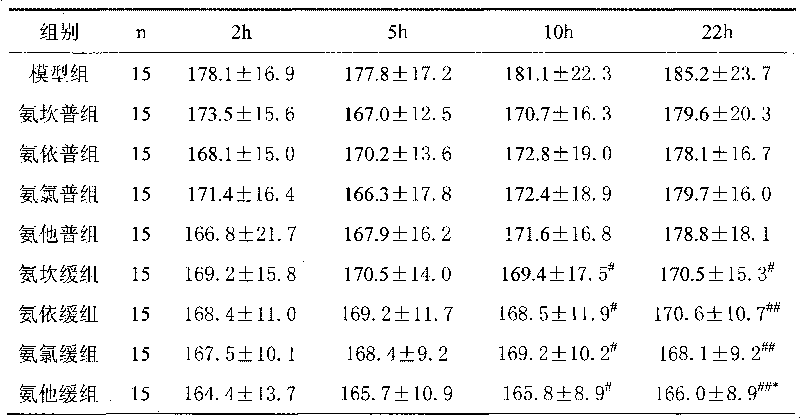
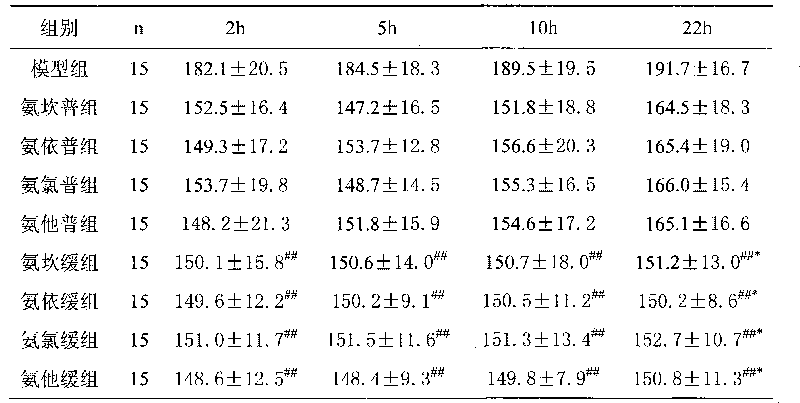
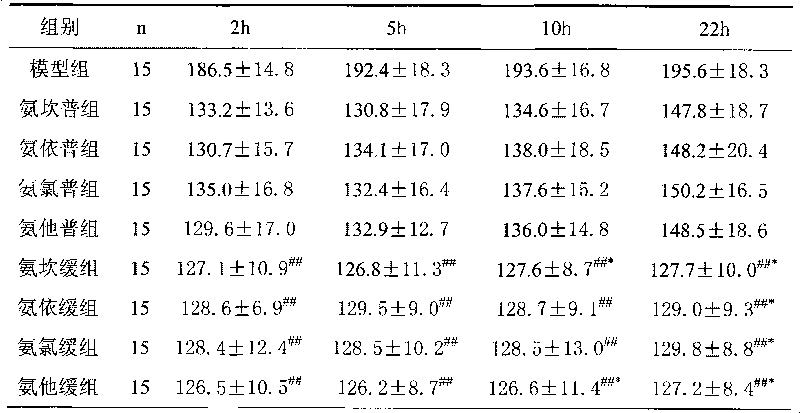
Examples
Embodiment 1
[0034] The preparation of embodiment 1 sustained release tablet
[0035] Levoamlodipine Besylate 5g
[0036] Candesartan cilexetil 5g
[0037] Hydroxypropyl Methyl Cellulose 8g
[0038] Lactose 12g
[0039] Microcrystalline Cellulose 15g
[0040] 70% ethanol solution of 3% PVPk30 appropriate amount
[0041] Magnesium stearate 0.6g
[0042] Preparation Process:
[0043] First pass levamlodipine besylate and candesartan cilexetil through a 100-mesh sieve; hydroxypropyl methylcellulose, microcrystalline cellulose, lactose, and dextrin pass through an 80-mesh sieve.
[0044] Weigh levamlodipine besylate, candesartan cilexetil, hydroxypropyl methylcellulose, lactose, and microcrystalline cellulose according to the prescription amount, mix them evenly, and add 70% ethanol solution of 3% PVPk30 to prepare Soft material, passed through 18-mesh sieve to granulate, wet granule is dried at 50-60°C, dry granule is granulated through 16-mesh sieve, added magnesium stearate, mixed ev...
Embodiment 2
[0045] The preparation of embodiment 2 sustained-release tablets
[0046] Levoamlodipine Besylate 2.5g
[0047] Candesartan cilexetil 4g
[0048] Hydroxypropyl Methyl Cellulose 8g
[0049] Microcrystalline Cellulose 15g
[0050] Lactose 12g
[0051] 80% ethanol solution appropriate amount
[0052] Magnesium stearate 0.5g
[0053] Preparation Process:
[0054] First pass levamlodipine besylate and candesartan cilexetil through a 100-mesh sieve; hydroxypropyl methylcellulose, microcrystalline cellulose, lactose, and dextrin pass through an 80-mesh sieve.
[0055] Weigh levamlodipine besylate, candesartan cilexetil, hydroxypropyl methylcellulose, lactose, and microcrystalline cellulose according to the prescription amount, mix them evenly, and add 70% ethanol solution of 3% PVPk30 to prepare Soft material, passed through 18-mesh sieve to granulate, wet granule is dried at 50-60°C, dry granule is granulated through 16-mesh sieve, added magnesium stearate, mixed evenly, and ...
Embodiment 3
[0056] The preparation of embodiment 3 sustained-release tablets
[0057] Levoamlodipine Besylate 5g
[0058] Eprosartan Mesylate 600g
[0059]Hydroxypropyl Methyl Cellulose 750g
[0060] Lactose 120g
[0061] Microcrystalline Cellulose 450g
[0062] Starch 300g
[0063] 80% ethanol solution of 3% PVPk30 appropriate amount
[0065] Preparation Process:
[0066] First pass levamlodipine besylate and eprosartan mesylate through a 100-mesh sieve; hydroxypropyl methylcellulose, microcrystalline cellulose, lactose, and starch pass through an 80-mesh sieve.
[0067] Weigh levamlodipine besylate, eprosartan mesylate, hydroxypropyl methylcellulose, lactose, microcrystalline cellulose and starch according to the prescription, mix them evenly, add 80% of 3% PVPk30 The ethanol solution is made of soft material, passed through a 18-mesh sieve to granulate, the wet granules are dried at 50-60°C, the dry granules are granulated through a 16-mesh sieve,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com