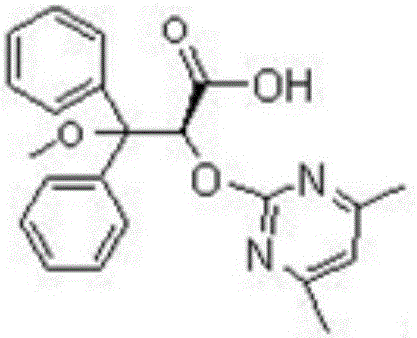
Preparation method of ambrisentan
A technology of ambrisentan and intermediates, applied in the field of compound preparation, can solve the problems of expensive resolving agent, high cost, large amount of organic solvent, etc., and achieve the effect of high resolution efficiency, low cost and low reagent toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
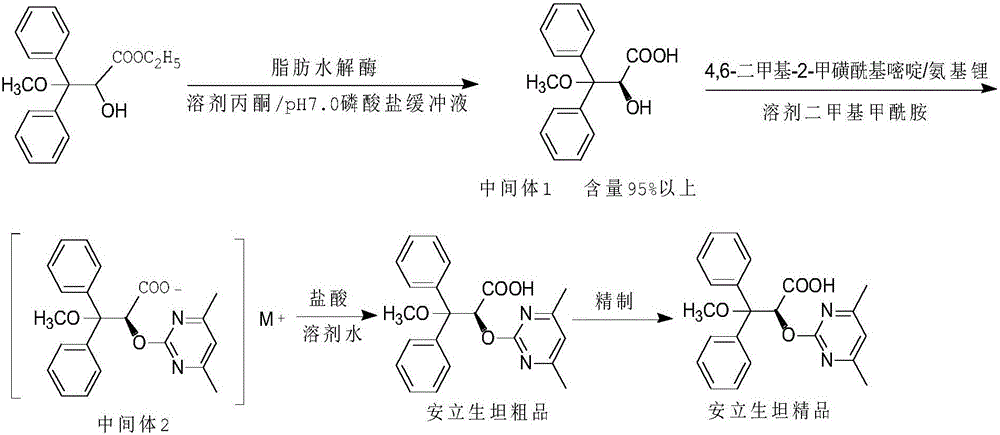
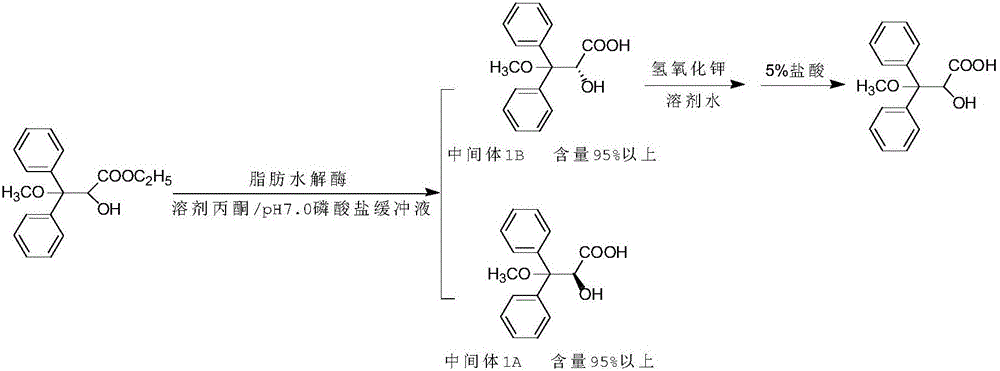
[0033] 1. Preparation of Intermediate 1
[0034] In a 500ml three-necked flask, add 240ml of N,N-dimethylformamide, 30g (0.1mol) of ethyl (R,S)-3,3-diphenyl-3-methoxy-2-hydroxypropionate, Lipase PS "Amano" SD 6g, pH7.0 phosphate buffer 90ml, phase transfer catalyst benzyltriethylammonium chloride 6g, reaction temperature 30°C, reaction time about 2h, HPLC monitoring, until S-configuration Less than 1% terminated the reaction. Add 100ml of 2N sodium carbonate solution to the reactant, stir for 30min, and separate the layers. The organic phases were combined, collected and concentrated to dry to obtain the R-configuration ester; the aqueous phase was washed once with 50ml of methyl tert-butyl ether, and then slowly added to 100ml of 3N hydrochloric acid under stirring, a large amount of white solids precipitated, stirred for 1h, filtered, and the solid The product was dried to obtain 12.5 g of S-configuration acid intermediate 1 with a yield of 41.7% and a chiral content of 97...
Embodiment 2
[0042] 1. Preparation of Intermediate 1
[0043] Into a 500ml three-necked flask, add 240ml acetone, 40g (0.13mol) of ethyl (R,S)-3,3-diphenyl-3-methoxy-2-hydroxypropionate, 5g lipase AP6, pH7 .0 Phosphate buffer 120ml, phase transfer catalyst tributylamine 5g, reaction temperature 40°C, reaction time about 2h, HPLC monitoring, S-configuration is less than 1% to terminate the reaction. Add 150ml of 2N sodium carbonate solution to the reactant, stir for 30min, and separate the layers. After the organic phase was racemized, it continued to participate in the reaction as a substrate; the aqueous phase was washed once with 60ml of methyl tert-butyl ether, and then slowly added to 150ml of 3N hydrochloric acid under stirring, a large amount of white solids precipitated, stirred for 1h, filtered, and dried 18.6 g of intermediate 1 was obtained by drying, the yield was 46.5%, and the chiral content was 98.2%.
[0044] 2. Preparation of Crude Ambrisentan
[0045] At room temperatur...
Embodiment 3
[0050] 1. Preparation of Intermediate 1
[0051] In a 500ml three-necked flask, add 320ml of ethanol, 40g (0.13mol) of ethyl (R,S)-3,3-diphenyl-3-methoxy-2-hydroxypropionate, lipohydrolase PS "Amano" IM 4g, pH 7.0 phosphate buffer 100ml, phase transfer catalyst chain polyethylene glycol 4g, reaction temperature 50°C, reaction time about 2h, HPLC monitoring, S-configuration is less than 1% to terminate the reaction. Add 150ml of 2N sodium carbonate solution to the reactant, stir for 30min, and separate the layers. The organic phases were combined, collected and concentrated to dry to obtain the R-configuration ester; the aqueous phase was washed once with 50ml of methyl tert-butyl ether, and then slowly added to 150ml of 3N hydrochloric acid under stirring, a large amount of white solids were precipitated, stirred for 1h, filtered, and dried 18.2 g of intermediate 1 was obtained, the yield was 45.5%, and the chiral content was 97.8%.
[0052] 2. Preparation of Crude Ambrisent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com