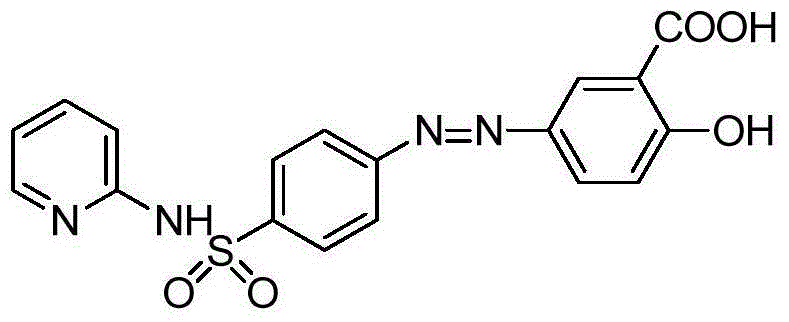
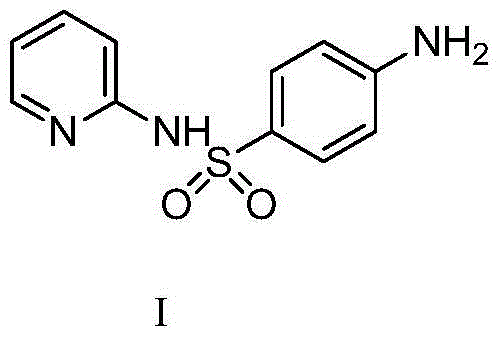
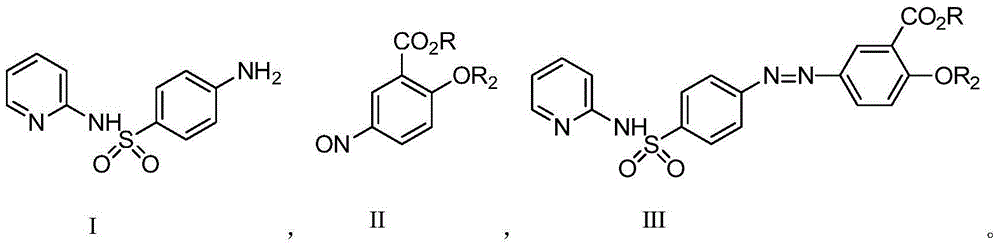
Sulfasalazine synthesis process
A technology of sulfasalazine and compounds, which is applied in the synthesis process of sulfasalazine and the field of sulfasalazine, and can solve problems such as low yield of sulfasalazine, easy hydrolysis of diazonium salt, and influence on reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of sulfasalazine
[0046]
[0047] Add the compound of formula I (2g, 8mmol) into 2-20ml of water at 20-25°C and stir, then add hydrochloric acid (1.62g 16mmol) and stir to dissolve, heat up to 30-35°C and keep it warm. Add the methanol (10-50ml) solution of Formula II (1.34g, 8mmol) dropwise at 30-35°C, and heat up to 45-50°C after dropping to complete the reaction. After heat preservation, it was cooled to 10-15°C for suction filtration, and the material was dried by suction to obtain 2.96 g of sulfasalazine with an HPLC purity of 98.9%.
Embodiment 2
[0048] Embodiment 2: the preparation of sulfasalazine
[0049]
[0050] Add the compound of formula I (2g, 8mmol) into 20ml of water at 20-25°C and stir, then add acetic acid (1.09g, 18mmol) and stir to dissolve, heat up to 30-35°C and keep warm. Add the methanol (20ml) solution of formula II (1.34g, 8mmol) dropwise at 30-35°C, and heat up to 45-50°C after dropping to complete the reaction. After the heat preservation is completed, cool to 10-15°C and filter with suction, dry the material and obtain 2.87 g of sulfasalazine.
Embodiment 3
[0051] Embodiment 3: the preparation of sulfasalazine
[0052]
[0053] The compound of formula I (2g, 8mmol) was added into acetic acid (2.9g, 48mmol) at 20-25°C and stirred, and the temperature was raised to 30-35°C. Add the methanol (20ml) solution of Formula II (1.34g, 8mmol) dropwise at 30-35°C. After the drop is complete, raise the temperature to 45-50°C and keep warm until the reaction is complete. After the heat preservation is completed, cool to 10-15°C and filter with suction, drain and dry the material to obtain 2.66 g of sulfasalazine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com