Polynucleotide constructs having disulfide groups
A technology of polynucleotides and constructs, applied in phosphorus organic compounds, organic chemistry, compounds of group 5/15 elements of the periodic table, etc., can solve problems such as cytotoxicity and ineffective delivery of transfection reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
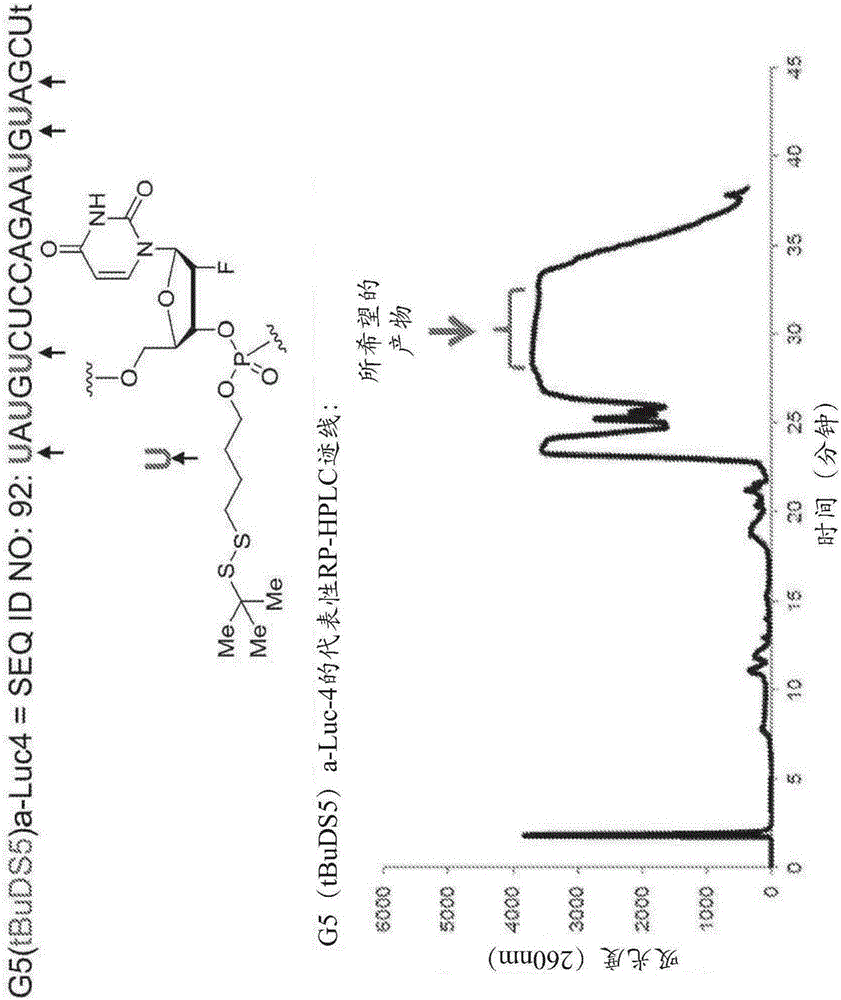
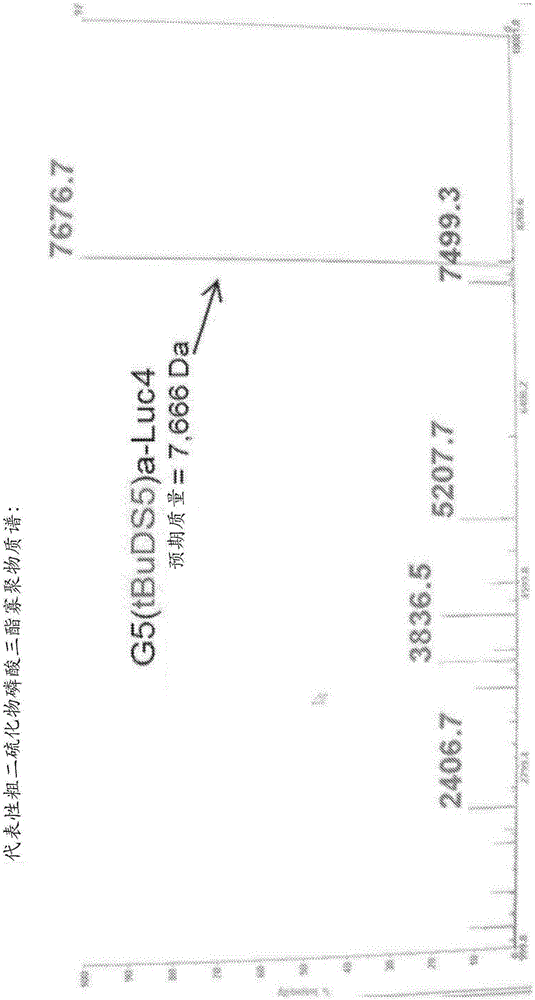
[0639] Example 1 Synthesis and purification of nucleotides and polynucleotides of the present invention
[0640] general synthesis program
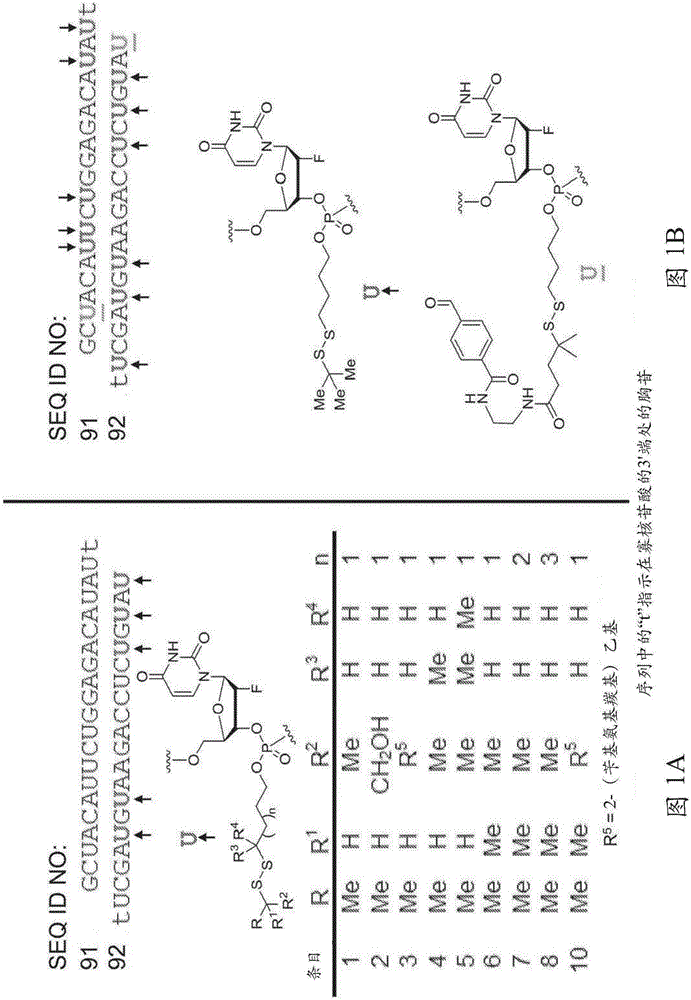
[0641] The polynucleotide constructs of the invention can be prepared according to the general and specific methods described herein. For example, a thiol-containing starting material undergoes a reaction with a 2,2'-bipyridyl disulfide to give the corresponding dipyridyl disulfide compound (see, for example, Scheme 1), which is then Disulfide compounds are subjected to reaction with nucleoside phosphoramidites to produce nucleotide constructs of the invention (eg, see Scheme 1). These nucleotide constructs are then used in standard oligonucleotide synthesis protocols to form polynucleotide constructs. These polynucleotide constructs were then deprotected and purified using HPLC.
[0642] plan 1
[0643]
[0644] Specific synthesis of nucleotides of the present invention
[0645] Exemplary syntheses of nucleotides of the invention...
example 2
[1273] Example 2 in vitro activity assay
[1274] Polynucleotides targeting the luciferase gene (GL3) were synthesized and used to generate polynucleotides with attached internucleotide bridging groups (phosphotriesters) and / or terminal groups (phosphodiesters) or phosphotriesters) of one or more disulfide-linked polynucleotide constructs.
[1275] To assess the in vitro activity of these disulfide phosphotriesters, human ovarian SKOV-3 cells stably expressing luciferase (GL3) were used. Cells were grown in McCoy's 5A medium (Life Technologies) supplemented with 10% fetal bovine serum (FBS), 100 μg / ml streptomycin, and 100 U / ml penicillin. Cells (1×10 4 / well) were seeded in 96-well microtiter plates and incubated at 37°C in 5% CO 2 Incubate overnight.
[1276] Controls: A control siRNA targeting the luciferase gene or a non-targeting control gene were transfected into cells at the indicated concentrations (typically 0.01-30 nM) using lipofectamine RNAiMax (Life Technologi...
example 3
[1298] Example 3 Cell Binding Experiment
[1299] Disulfide phosphotriester oligonucleotide-Cy3 cell binding general protocol: Incorporate a core of the invention containing a disulfide group attached to one or more internucleotide bridging groups and / or terminal groups The nucleotide construct anneals to G at a final concentration of 10 mM 2’Mod -Cy3 (guide strand).
[1300] Cell Treatment Setup: Seed 40,000 cells per well in a 48-well plate; allow cells to adhere overnight. Then, cells were washed once with 500 μl of PBS, and then 150 μl was added for treatment (note: for free folic acid samples, cells were treated with medium containing 2.3 mM folic acid for 1 hour prior to treatment). Cells were treated for 4 hours; after 4 hours, cells were washed once with PBS, trypsinized and analyzed by flow cytometry for siRNA-Cy3 cell association.
[1301] The results of these experiments are in Figure 9 A. Figure 9 B. Figure 10 A. Figure 10 B. Figure 11 A. and Figure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com