Antitumor pharmaceutic preparation composition
A technology of anti-tumor drugs and preparations, applied in the directions of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of metastasis, tumor recurrence, and tumor cells are not effectively killed, so as to reduce side effects, eliminate tumor cells, The effect of reducing clinical dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
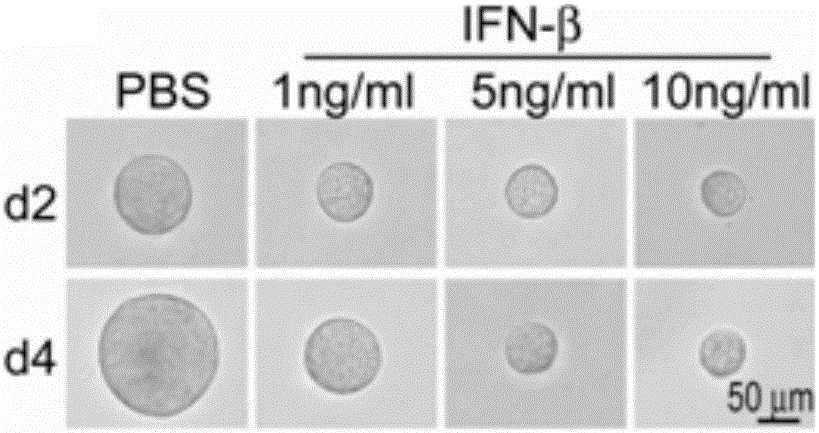
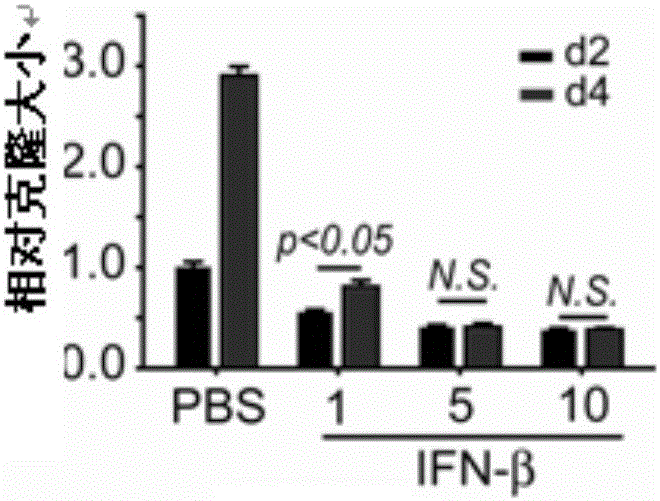
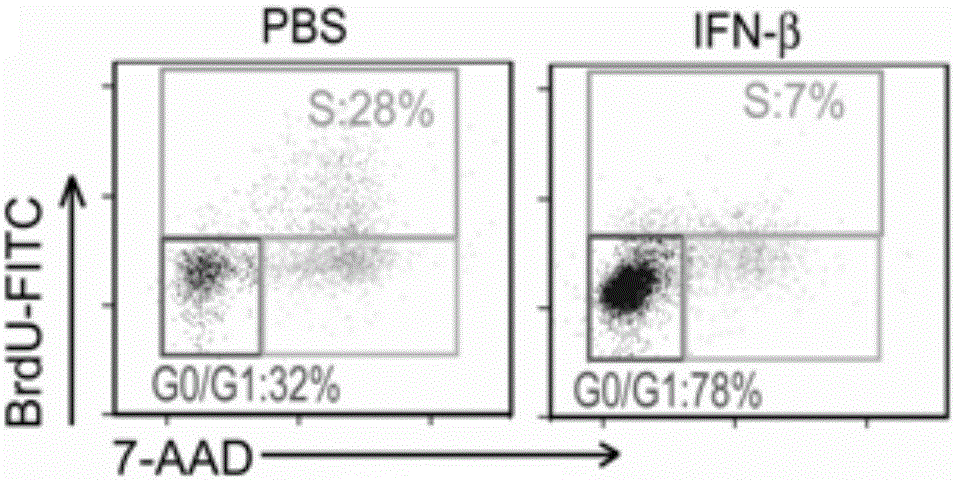
[0033] Example 1: IFN-β causes B16 melanoma cells to enter dormancy.
[0034] 1. Experimental steps:
[0035] Control group (PBS): 3000 B16 tumor cells were planted in 3D fibrin soft gel on a 24-well plate (the gel volume in a single gel-containing well was 250 μL, and ordinary medium (DMEM) added on the gel Culture medium) is 1 mL), and cultured for 3-5 days with ordinary medium plus PBS (the volume added is very small, only 1 microliter);
[0036]Experimental group (IFN-β): 3000 B16 tumor cells were planted in 3D fibrin soft gel, cultured with ordinary medium for 2 days, and then replaced with ordinary medium + IFN-β medium, The concentration of IFN-β was 1 ng / mL, 5 ng / mL or 10 ng / mL respectively. After stimulating B16 tumor cells for 2-4 days, observe the cell growth, perform cell cycle measurement, collect cell supernatants at different time points to measure glucose concentration, And use the principle of SA-β-gal to judge whether IFN-β causes B16 tumor cell senescence ...
Embodiment 2
[0044] Example 2: Inhibition of IDO signaling pathway reverses tumor dormancy caused by IFN-β (in vitro cell experiment of the combination of anti-tumor drug preparations of the present invention).
[0045] 1. Experimental steps:
[0046] Control group 1 (PBS): 3000 normal B16 tumor cells were planted in a 3D fibrin soft gel on a 24-well plate (the volume of the gel in a single gel-containing well was 250 μL, and the normal medium added on the gel 1 mL), cultured for 2-4 days with ordinary medium+PBS (the volume added is only 1 microliter);
[0047] Control group 2 (IFN-β): 3000 B16 tumor cells were planted in 3D fibrin soft gel, and after being cultured in ordinary medium for 2 days, IFN-β (5ng / mL) was added to ordinary medium to treat 2- 4 days;
[0048] Experimental group: 3000 B16 tumor cells were planted in 3D fibrin soft gel. After culturing in common medium for 2 days, IFN-β (5ng / mL) and 1-MT (0.2mM or 0.5 mM) to treat cells for 2-4 days, or add IFN-β and DMF (20 μM ...
Embodiment 3
[0053] Example 3: Combined application of IFN-β and IDO inhibitors, or combined application of IFN-β and AhR inhibitors can kill tumor cells, especially dormant tumor cells.
[0054] 1. Combined application of IFN-β and IDO inhibitors (see Figure 3A )
[0055] 1) Experimental steps
[0056] Construction of tumor-bearing mice: 40 C57BC / L mice at 4-6 weeks were randomly divided into 4 groups with a weight of 20 g; each mouse was subcutaneously inoculated with 1×10 4 B16 melanoma tumor cells, 15 days later, the tumor grew to 7 mm × 7 mm, and the tumor-bearing mice were given the following different treatments on the 16th day:
[0057] Control group 1 (PBS): only intratumoral injection of PBS to tumor-bearing mice every day;
[0058] Control group 2 (IFN-β): 250ng IFN-β (500U / ng) was injected intratumorally into each tumor-bearing mouse, once every three days, for a total of 3 injections;
[0059] Control group 3 (1-MT): 1-MT was dissolved in the drinking water of tumor-beari...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com