Acridine marker conjugate, preparation method of acridine marker conjugate and chemical luminescent kit
A conjugate and acridine technology, applied in the field of chemiluminescent kits, acridine-labeled conjugates and their preparation, can solve the problems of reduced activity of acridine-labeled conjugates and affecting the sensitivity of immunoassays, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
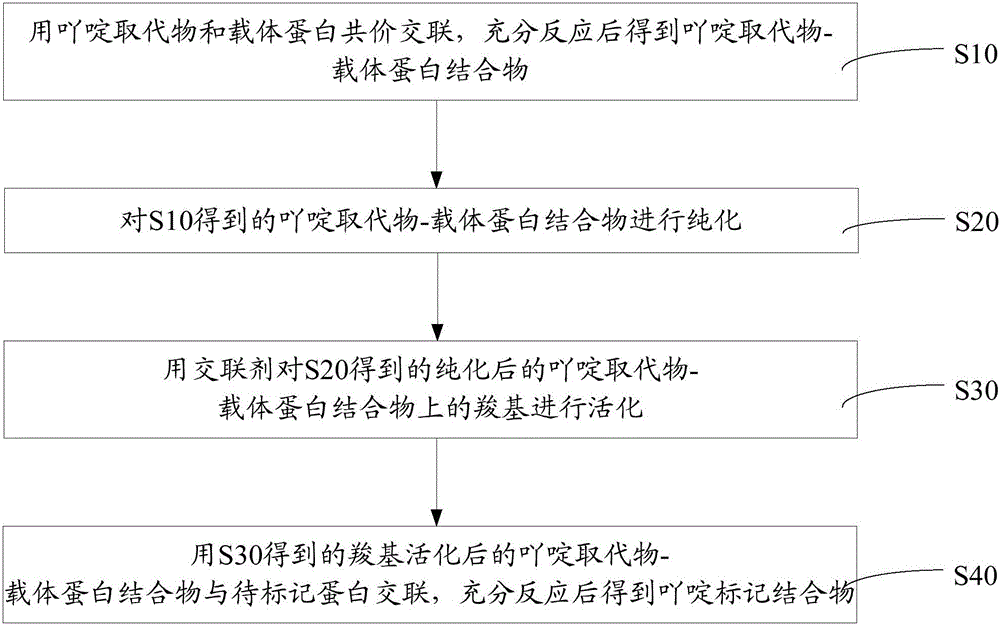
[0047] Such as figure 1 The preparation method of the above-mentioned acridine-labeled conjugate shown includes the following steps:
[0048] S10, covalently cross-linking the acridine substituent with the carrier protein, and obtaining the acridine substituent-carrier protein conjugate after sufficient reaction.
[0049] In the operation of covalently cross-linking the acridine substituent and the carrier protein, the molar ratio of the acridine substituent to the carrier protein is 100-20000:1.
[0050] Preferably, the molar ratio of the acridine substituent to the carrier protein is 500-5000:1.
[0051] The carrier protein is a protein, modified protein, polypeptide or modified polypeptide containing carboxyl and amino groups, and the carrier protein is connected by a chemical bond through the reaction of the amino group on the carrier protein with the acridine substituent.
[0052] The carrier protein can be a protein or polypeptide that itself has carboxyl and amino gro...
Embodiment 1
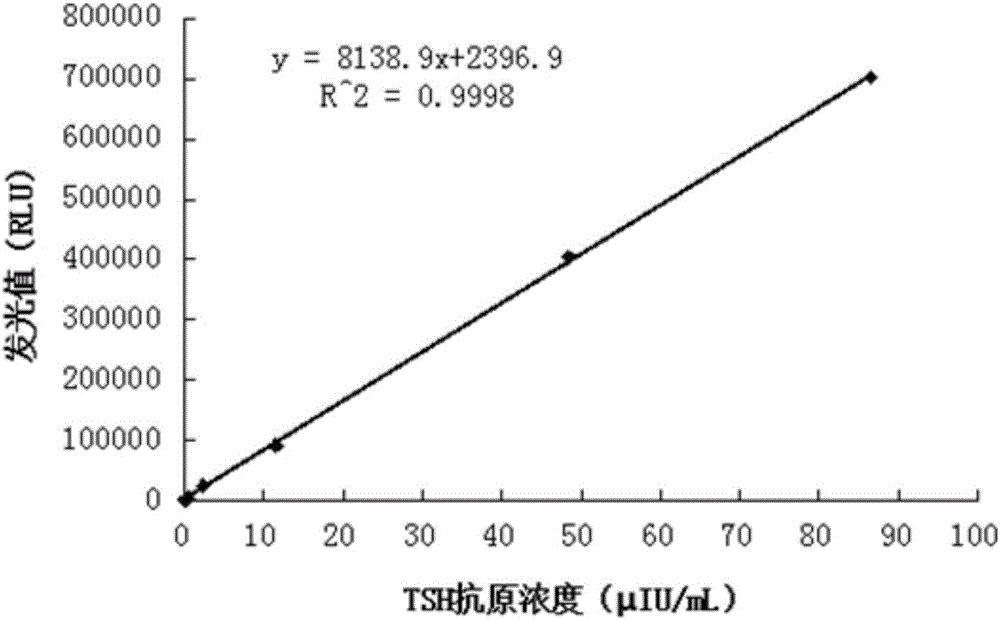
[0098] Dissolve 1 mg BSA in 1 mL of 150 mM PBS buffer (pH 7.4), add 40 μL of acridinium ester (10 mg / mL dissolved in DMF) and react at 25°C for 4 h, use 5 mL of 7KD molecular weight cut-off desalting column (Thermofish Company) to dilute with 150 mM PBS ( pH 7.4) buffer solution was used as the replacement buffer solution, and passed through the column for 3 times to remove free acridinium esters and reaction by-products to obtain an acridinium-BSA solution.
[0099] EDC (final concentration: 10 mmol / L) and NHS (final concentration: 20 mmol / L) were added to the above-mentioned purified acridine-BSA solution. After reacting at 25°C for 10 minutes, TSH antibody (manufacturer: Santa Cruz biotechnology, product number: sc-418393) was added, mixed evenly, and placed at 25°C for 4 hours, and 5 mL of a 7KD molecular weight cut-off desalting column (Thermofish Company) was used to dilute with 150 mM PBS ( pH 7.4) buffer was used as the replacement buffer, and the column was passed 3 t...
Embodiment 2
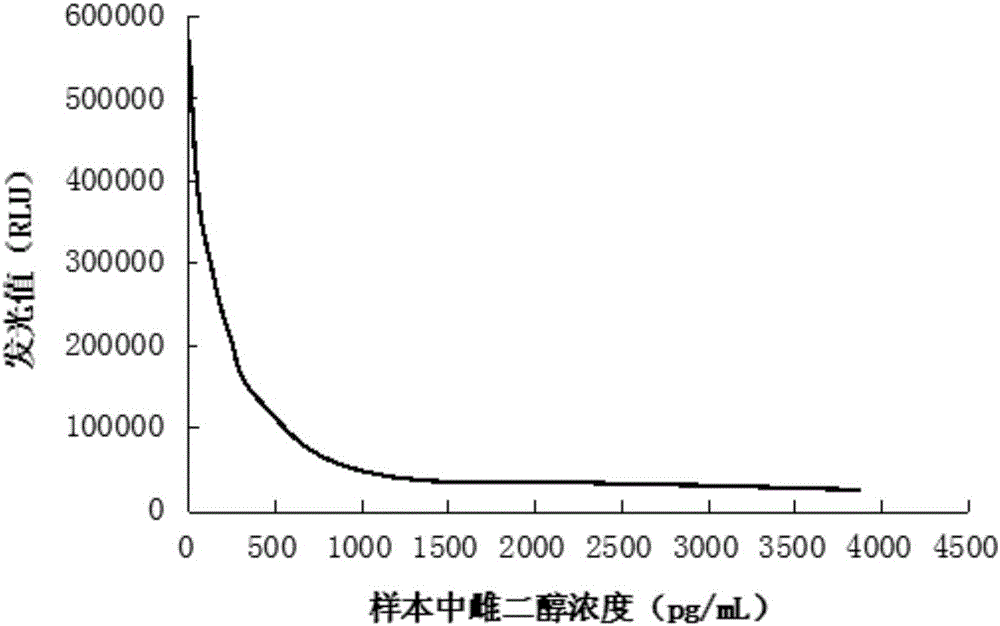
[0101] Dissolve 1mg BSA in 1mL 150mM PBS buffer (pH 7.4), add 40μL acridinium ester (10mg / mL dissolved in DMF) and react at 25°C for 4h, use 5mL 7KD molecular weight cut-off desalting column (Thermofish Company) (pH 7.4) buffer solution was used as the replacement buffer solution, and the column was passed 3 times to remove free acridinium esters and reaction by-products to obtain an acridinium-BSA solution.
[0102] EDC (final concentration: 10 mmol / L) and NHS (final concentration: 20 mmol / L) were added to the above-mentioned purified acridine-BSA solution. After reacting at 25°C for 10 minutes, add estradiol antigen (manufacturer: abcam, product number: ab120657), mix well, place at 25°C for 4 hours, and use 5mL 7KD molecular weight cut-off desalting column (Thermofish Company) to dissolve 150mM PBS (pH 7.4 ) buffer as a liquid replacement buffer, passed through the column 3 times to remove free EDC, NHS and reaction by-products to obtain an acridinium ester-labeled estradio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com