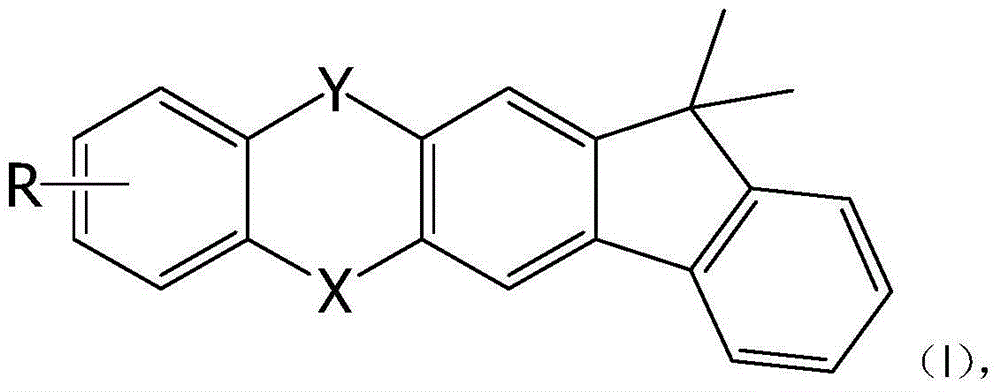
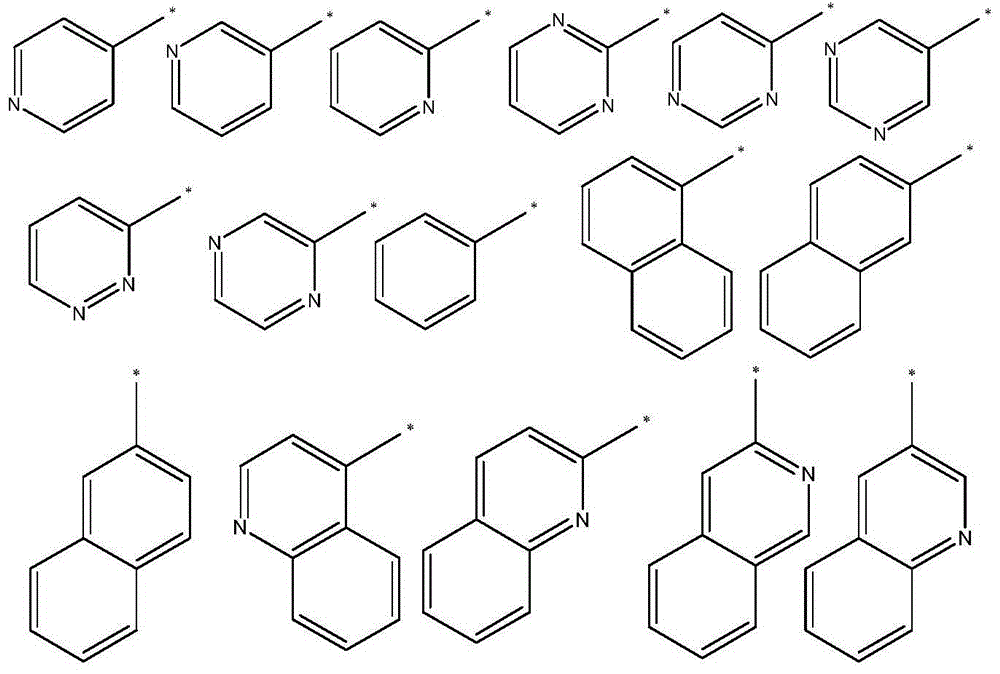
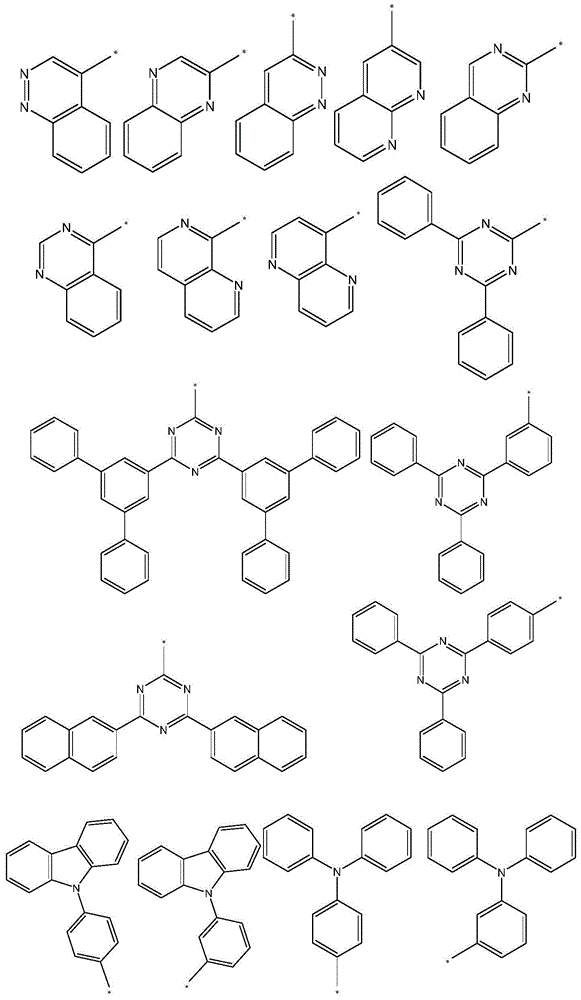
Indeno heteroanthracene compound and application thereof
A technology of heterocyclic compounds and compounds, which is applied in the field of compounds, can solve the problems of low Tg, easy crystallization of small molecule host materials, etc., and achieve the effects of increasing Tg, reducing crystallization, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation process
[0032]
[0033] 2. Preparation method
[0034] The first step: under nitrogen protection, charge 3-hydroxy-9,9-dimethylfluorene (22.1g, 0.11mol , 1.1eq), 4-bromo-3-nitrobiphenyl (27.7g, 0.1mol, 1.0eq), K2CO3 (31.8g, 0.3mol, 3.0eq), then 1L of acetone was added to the above mixture, and then the circle The mixture in the bottom flask was reacted at 60°C for 16 hours and then cooled to room temperature.
[0035] The contents in the above-mentioned cooled flask were spin-dried, and 600mL deionized water and 600mL ethyl acetate were added to the residue, stirred at 30°C for 30 minutes, then left to stand, and after the organic phase and the aqueous phase were separated, the The two separate. The separated organic phase was successively washed with water 3 times (600 mL each time) and saturated brine 3 times (600 mL each time). The washed organic phase was dried with anhydrous sodium sulfate, filtered and spin-dried, and the resulting substanc...
Embodiment 2
[0047] 1. Preparation process
[0048]
[0049] 2. Preparation method
[0050] The first step: Using the same reaction conditions as the first step of CPD 1, compound 1 and compound 5 were reacted under certain conditions to obtain compound 6 (33.59 g, yield: 82%).
[0051]The second step: using the same reaction conditions as the second step of CPD 1, compound 6, triethyl phosphite and 1,2-dichlorobenzene were reacted under certain conditions to obtain compound 7 (13.5g, yield: 72%).
[0052] The third step: using the same reaction conditions as the third step of CPD 1, compound 7 and 4-bromopyridine were reacted under certain conditions to obtain CPD 2 (5.92 g, yield: 65.7%).
[0053] 3. Products
[0054] Among them, the relevant characterization data of the product CPD 2 are as follows:
[0055] MS: m / z=454.19 (M+H+); 1HNMR (400M, d-CDCl3): 8.67 (d, 2H), 8.45 (d, 2H), 7.55-7.85 (m, 4H), 7.25-7.38 (m ,3H),6.65-6.98(m,6H),1.67(s,6H);
[0056] Elemental analysis: C31H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com