Hydrochloride crystal form B of Lu AE58054 and preparation method for hydrochloride crystal form B
A hydrochloride and crystal form technology, which is applied in the crystal form B of LuAE58054 hydrochloride and its preparation field, can solve the problems of reporting the crystal form of LuAE58054 hydrochloride, etc., and achieve convenient long-term storage and placement, simple operation, and hygroscopicity low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation method of Lu AE58054 hydrochloride crystal form B:
[0051] Dissolve 10.3 mg of Lu AE58054 hydrochloride in 25 μL of methanol and slowly add dropwise to 1.5 mL of methyl tert-butyl ether. A solid precipitates out. Stir at room temperature for 24 hours, filter and dry, and collect the solid. It is the crystalline form B of Lu AE58054 hydrochloride.
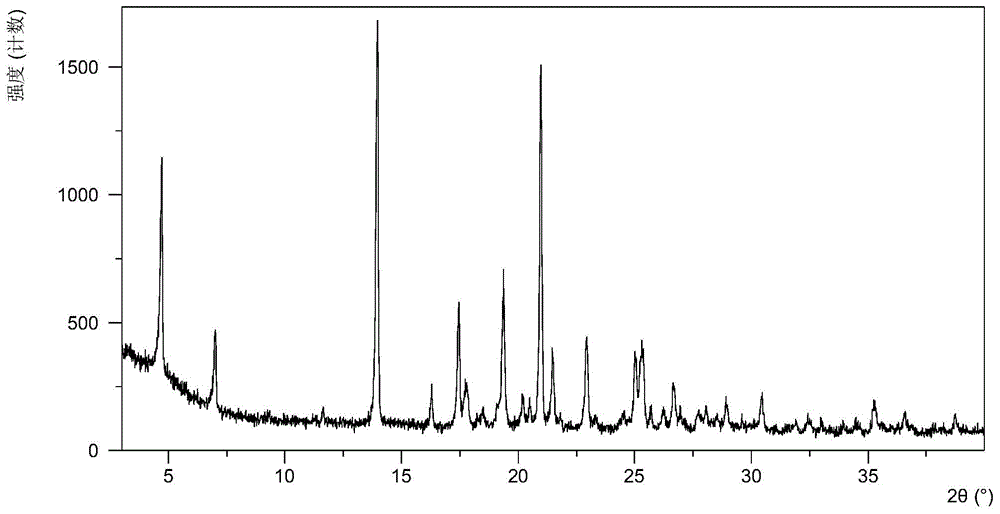
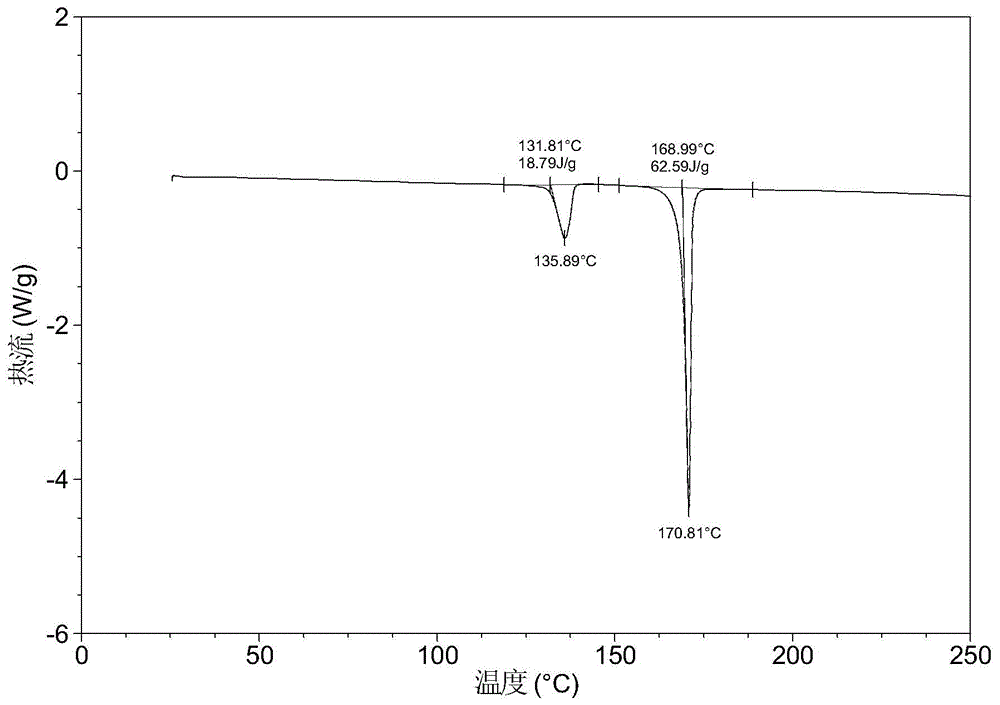
[0052] The X-ray powder diffraction data of the crystal form obtained in this example is shown in Table 1. Its XRPD diagram is like figure 1 , Its DSC graph is like figure 2 .
[0053] Table 1
[0054] 2theta d interval strength% 4.5919.2660.15 6.8912.8430.23 9.199.622.19 13.866.39100.00 16.185.488.15 17.345.1114.27 17.705.014.57 19.274.6118.14 20.874.2686.14 21.414.159.34 22.843.8913.29 25.203.5313.87 26.573.355.83 27.843.201.45 28.853.102.88 30.342.956.68 32.362.772.10 32.892.723.47 35.182.554.69 36.482.464.38 38.672.332.28
Embodiment 2
[0056] Preparation method of Lu AE58054 hydrochloride crystal form B:
[0057] 2.0 mg of Lu AE58054 hydrochloride was dissolved in 50 μL of methanol, and it was volatilized at room temperature until a solid precipitated. The solid was collected, and it was detected as Lu AE58054 hydrochloride crystal form B.
[0058] The X-ray powder diffraction data of the crystal form obtained in this example is shown in Table 2.
[0059] Table 2
[0060] 2theta d interval strength% 4.5819.2858.03 6.8912.8425.48 11.517.692.82 13.856.39100.00 16.185.487.79 17.345.127.05 17.675.022.76 18.394.821.49 19.284.6013.00 20.134.412.24 20.864.2672.46 21.374.167.20 22.853.8910.56 23.213.832.66 24.943.575.93 25.203.535.14 25.593.481.97 26.153.411.87 26.593.355.03 27.973.191.51 30.352.955.97 32.342.771.83 35.182.553.92 36.502.463.83 38.662.332.24
Embodiment 3
[0062] Preparation method of Lu AE58054 hydrochloride crystal form B:
[0063] 2.0 mg of Lu AE58054 hydrochloride was dissolved in 20 μL of acetone, and it was volatilized at room temperature until a solid precipitated. The solid was collected, and it was detected as the crystalline form B of Lu AE58054 hydrochloride.
[0064] Table 3 shows the X-ray powder diffraction data of the crystal form obtained in this example.
[0065] table 3
[0066] 2theta d interval strength% 4.5819.3045.51 6.8912.8328.26 11.517.694.20 13.856.40100.00 16.165.497.61 17.355.113.62 19.294.605.94 20.854.2677.27 21.374.163.59 22.833.904.06 23.213.832.72 24.923.577.04 26.573.355.72 27.583.231.87 27.963.192.11 30.332.958.15 32.352.771.25 35.162.553.62 36.472.462.66 38.662.331.41
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com