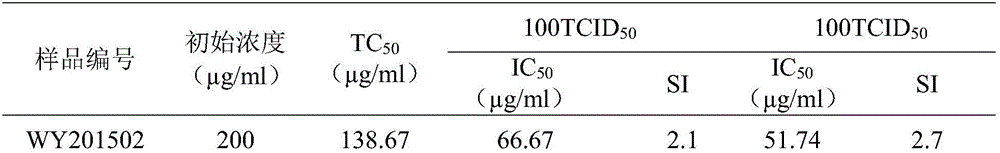Application of Zukamu granules in preparing medicine for treating virus flu
The technology of wood granules and zucchini is applied to zucchini granules and its application field in the preparation of drugs for treating viral influenza, which can solve problems such as no specific antiviral drugs, and achieve good therapeutic effects, strict structure, Damage reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Principle: MDCK (dog kidney) cells are used as the virus host, and the degree of inhibition of the virus-induced cytopathic effect (CPE) of the sample is determined.
[0042] 2 Materials and methods:
[0043] 1. Virus strain: Influenza virus A / Hanfang / 359 / 95 (H3N2), cultured and passaged in the allantoic cavity of chicken embryos (2013.9), stored at -80°C.
[0044] 2. Sample treatment: Before the sample is used, DMSO is made into a mother solution, and then diluted 3 times with the culture solution, each with 8 dilutions.
[0045] 3. Blank control drug: Xinjiang Uygur Pharmaceutical Co., Ltd.
[0046] 4. Test method: MDCK cells were inoculated in 96-well culture plate, placed in 5% CO 2 , cultivated at 37°C. Influenza virus 1 / 2 10 after 24 hours -5 , adsorb for 2 hours, discard the virus solution, add the maintenance solution containing samples of different dilutions and blank control drugs, and set up cell control wells and virus control wells at the same time, ...
Embodiment 2
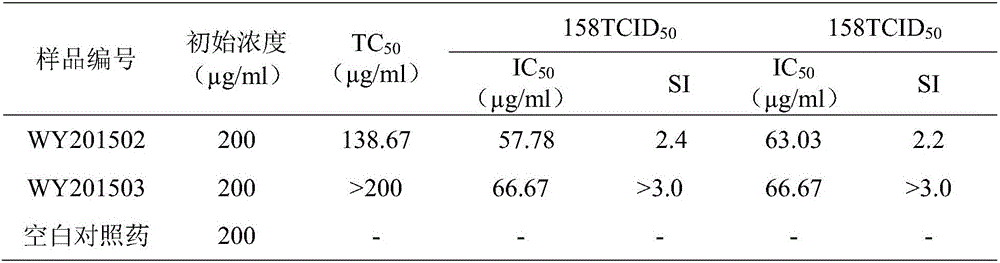
[0056] 1. Principle: MDCK (dog kidney) cells are used as the virus host, and the degree of inhibition of the virus-induced cytopathic effect (CPE) of the sample is determined.
[0057] 2 Materials and methods:
[0058] 1 Virus strain: Influenza virus A / Guangdong Luohu / 219 / 2006 (H1N1), cultured and passaged in the allantoic cavity of chicken embryos (2013.9), stored at -80°C.
[0059] 2. Sample treatment: Before the sample is used, DMSO is made into a mother solution, and then diluted 3 times with the culture solution, each with 8 dilutions.
[0060] 3 Blank control drug: Xinjiang Uygur Pharmaceutical Co., Ltd.
[0061] 4 Test method: MDCK cells were inoculated in 96-well culture plate, placed in 5% CO 2 , cultivated at 37°C. Influenza virus 1 / 2 10 after 24 hours -4 , adsorb for 2 hours, discard the virus solution, add maintenance solutions containing samples of different dilutions and blank control drugs, set up cell control wells and virus control wells, and incubate at 5...
Embodiment 3
[0070] 1. Principle: MDCK (dog kidney) cells are used as the virus host, and the degree of inhibition of the virus-induced cytopathic effect (CPE) of the sample is determined.
[0071] 2 Materials and methods:
[0072] 1 Virus strain: Influenza virus A / Jiangxi Donghu / 312 / 2006 (H3N2), cultured and passaged in the allantoic cavity of chicken embryos (2013.9), stored at -80°C.
[0073] 2. Sample treatment: Before the sample is used, DMSO is made into a mother solution, and then diluted 3 times with the culture solution, each with 8 dilutions.
[0074] 3 Blank control drug: Xinjiang Uygur Pharmaceutical Co., Ltd.
[0075] 4 Test method: MDCK cells were inoculated in 96-well culture plate, placed in 5% CO 2 , cultivated at 37°C. Influenza virus 1 / 2 10 after 24 hours -4 , adsorb for 2 hours, discard the virus solution, add the maintenance solution containing samples of different dilutions and blank control drugs, and set up cell control wells and virus control wells at the same ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com