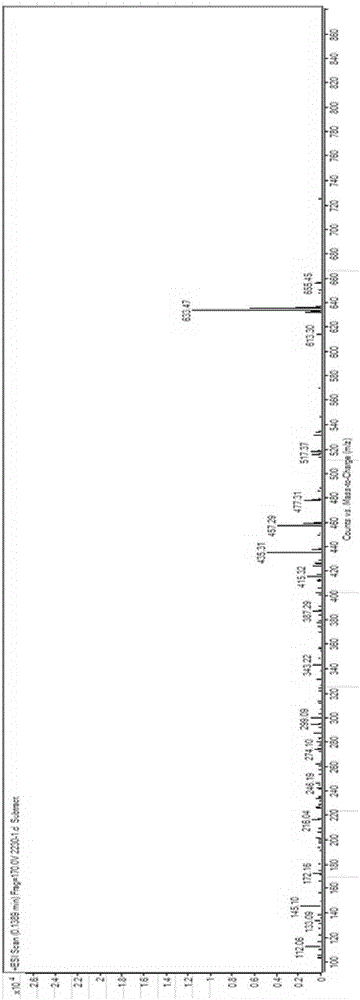
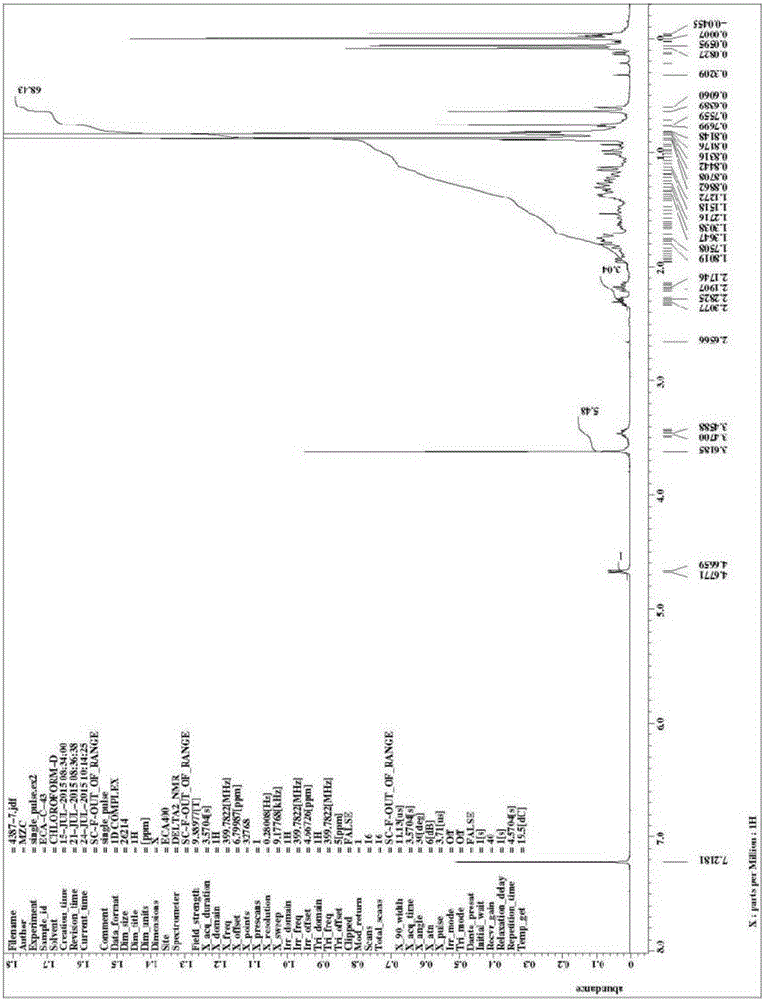
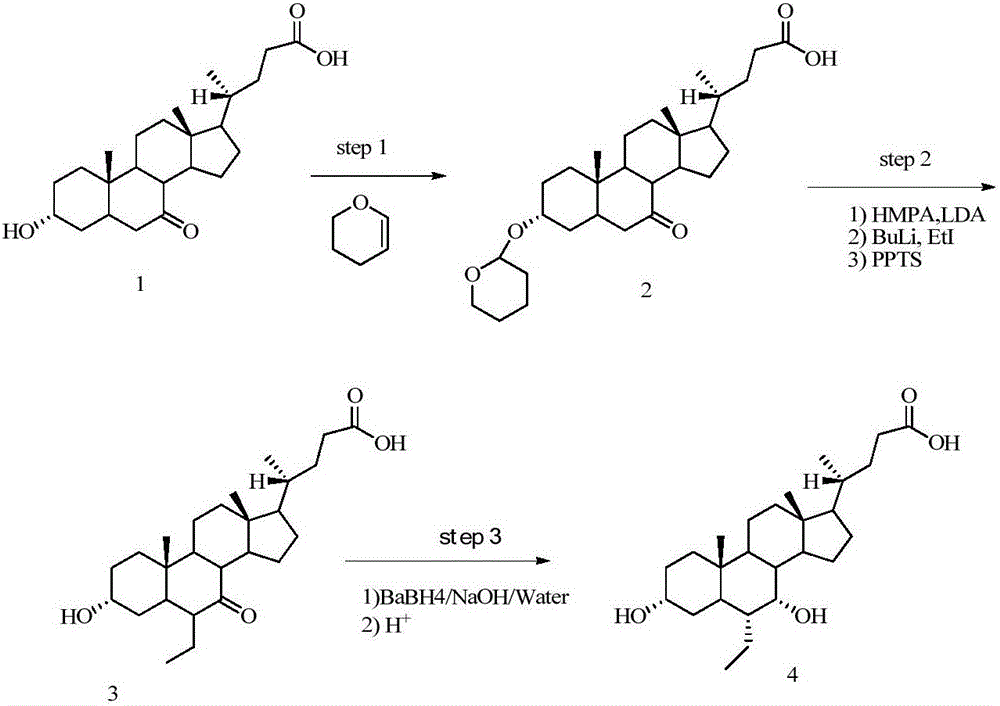
3,7-di(t-butyldimethylsiloxy)-6-ene-5beta-cholan-24-oic acid methyl ester
A technology of tert-butyldimethylsilyloxy and acid methyl ester, which is applied in the field of medicine and can solve problems such as inconvenient use, unstable properties of trimethylsilyl ether groups, and influence on the yield and purity of the final product.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0106] Embodiments of the present invention will be described in detail below in conjunction with the drawings and examples, but those skilled in the art will understand that the following drawings and examples are only for illustrating the present invention, and should not be considered as limiting the scope of the present invention. Those who do not indicate the specific conditions in the accompanying drawings and the examples are carried out according to the conventional conditions or the conditions suggested by the manufacturer. The reagents or instruments used were not indicated by the manufacturer, and they were all commercially available conventional products. All anhydrous solvents used are commercially available.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com