Process for fluorinating end groups of perfluoropolyether acids
A technology of perfluoropolyether acid and perfluoropolyether, which is applied in the field of fluorine chemical synthesis, can solve the problems of low fluorination conversion rate and low utilization rate of fluorinating agent gas, and achieve less by-products, safe and stable reaction, and fluorine The effect of chemical reaction is sufficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
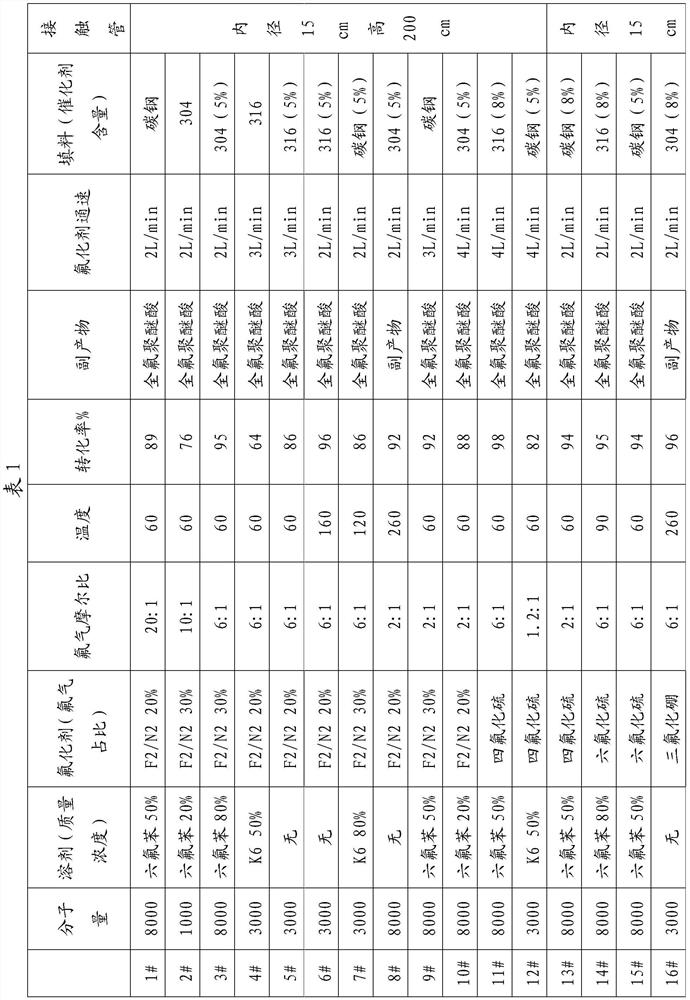
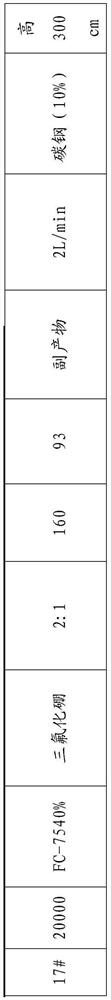
[0021] Fill the gas-liquid contact reaction tube with an inner diameter of 15 cm and a height of 200 cm with fillers passivated by fluorine gas with a particle size of 3 mm. The fillers in this example are carbon steel, and the temperature is controlled at 60 ° C to carry out the reaction of fluorinated perfluoropolyether acids , taking fluorine nitrogen gas with a fluorine gas content of 20% as the fluorinating agent. Hexafluorobenzene is used as a solvent, and a perfluoropolyether acid solution with a mass concentration of 50% and a weight average molecular weight of 8000 g / mol is used as a reaction solution. The fluorination experiment was carried out by controlling the mole of fluorine gas in fluorine nitrogen to be 20:1 of perfluoropolyether. Blow the fluorinating agent into the air inlet at the reaction bottom of the catalytic tube at a speed of 2L / min, and the product perfluoropolyether content collected in the product collection bottle at the lower end of the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com