Amide derivatives of 1-oxa-4,9-diazaspiro undecane compounds having multimodal activity against pain
A compound, alkyl technology, applied in the field of pain treatment, diazaspiroundecane derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment D
[0162] In Example DA, the following conditions apply:
[0163] when Y is where R 3 and R 3' for hydrogen, R 1 for C(O)R 5 and X is not -C(R 4 R 4' )-, then n will be 2.
[0164] In Example DA, the following compounds are preferably excluded:
[0165] and / or
[0166] and / or
[0167] and / or
[0168]
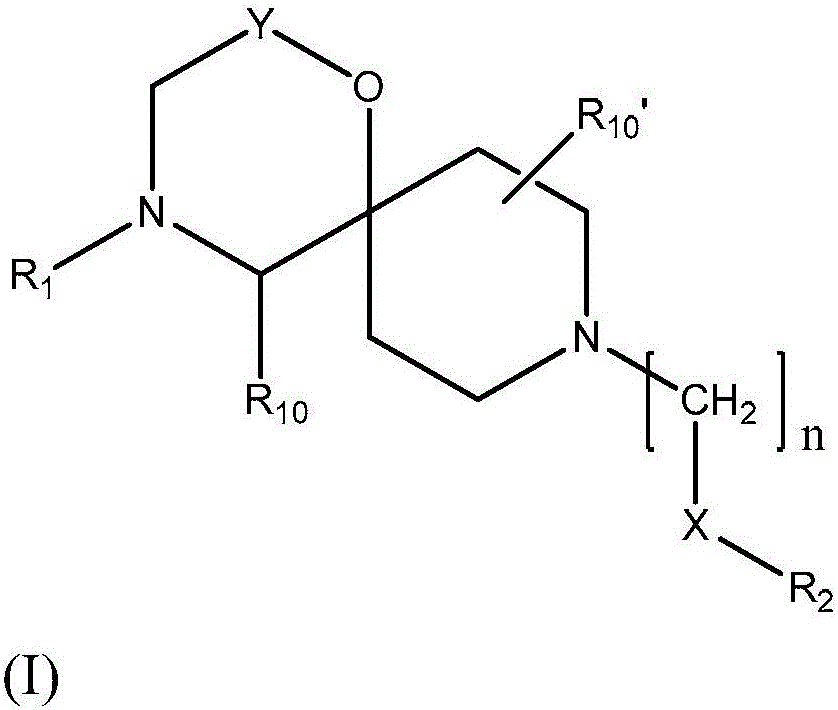
[0169] In one embodiment (Example DB), the compound is a compound of general formula (I),
[0170]
[0171] in
[0172] Y is
[0173] n is 1 or 2;
[0174] q is 1, 2, 3, 4, 5 or 6;
[0175] X is a bond, -C(O)O-, -C(O)NR 8 -, -C(O)- or -O-;
[0176] R 1 for C(O)R 5 or S(O) 2 R 5 ;
[0177] R 2 is substituted or unsubstituted C 1-6 Alkyl, substituted or unsubstituted C 2-6 Alkenyl, substituted or unsubstituted C 2-6 Alkynyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted aryl or substituted or unsubstituted heterocyclyl; wherein cycloalkyl, aryl or heterocyclyl (if substituted ) is substituted with a substituent se...
Embodiment DB
[0188] In the example DB, the following conditions apply:
[0189] where Y is and
[0190] -(CH 2 ) n -X-R 2 is an alkyl group, the alkyl group contains 6 or less C atoms.
[0191] In the example DB, the following conditions apply:
[0192] when Y is where R 3 and R 3' is hydrogen, and R 1 for C(O)R 5 , then n will be 2.
[0193] In Example DB, the following compounds are preferably excluded:
[0194]
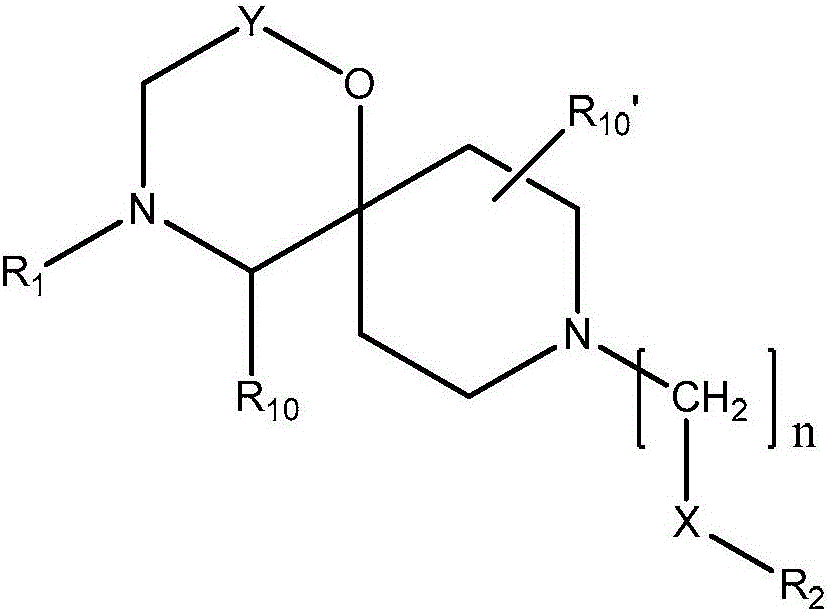
[0195] In one embodiment (Example DC), the compound is a compound of general formula (I),
[0196]
[0197] in
[0198] Y is
[0199] n is 1 or 2;
[0200] q is 1, 2, 3, 4, 5 or 6;
[0201] X is a bond, -C(O)O-, -C(O)NR 8 -, -C(O)- or -O-;
[0202] R 1 for C(O)R 5 or S(O) 2 R 5 ;
[0203] R 2 is substituted or unsubstituted C 1-4 Alkyl, substituted or unsubstituted C 2-4 Alkenyl, substituted or unsubstituted C 2-4 Alkynyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted aryl or substituted or unsubstituted heterocyclyl;...
example
[0836] General experimental part (methods and equipment for synthesis and analysis)
[0837] Process 1
[0838] A 2-step process for the preparation of compounds of general formula (I) starting from compounds of formula II is described, as shown in the following scheme:
[0839]
[0840] Process 1
[0841] where R 1 , R 2 , R 10 , R 10' , n, Y and X have the meanings defined above for the compound of formula (I), p represents 0, 1 or 2, LG represents a leaving group such as halogen, mesylate, tosylate or Triflate, p represents a suitable protecting group (preferably Boc), and P' represents another suitable protecting group (preferably 4-methoxybenzyl or benzyl) and Z represents COOH, COW or SO 2 W, wherein W represents a halogen.
[0842] The 2-step method was performed as follows:
[0843] Step 1: The reduction reaction of the compound of formula II to produce the compound of formula III can use a suitable reducing agent such as lithium aluminum hydride, borane-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com