Application of 1-pyrenecarboxylic acid as pH fluorescence probe
A fluorescent probe, pyrene formic acid technology, applied in the field of optical analysis and detection, can solve the problems of low probe sensitivity, wide response range, discomfort, etc., and achieve the effects of strong selectivity, wide application range and good anti-interference ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Fluorescence recognition of 1-pyrene acid for different pH values:
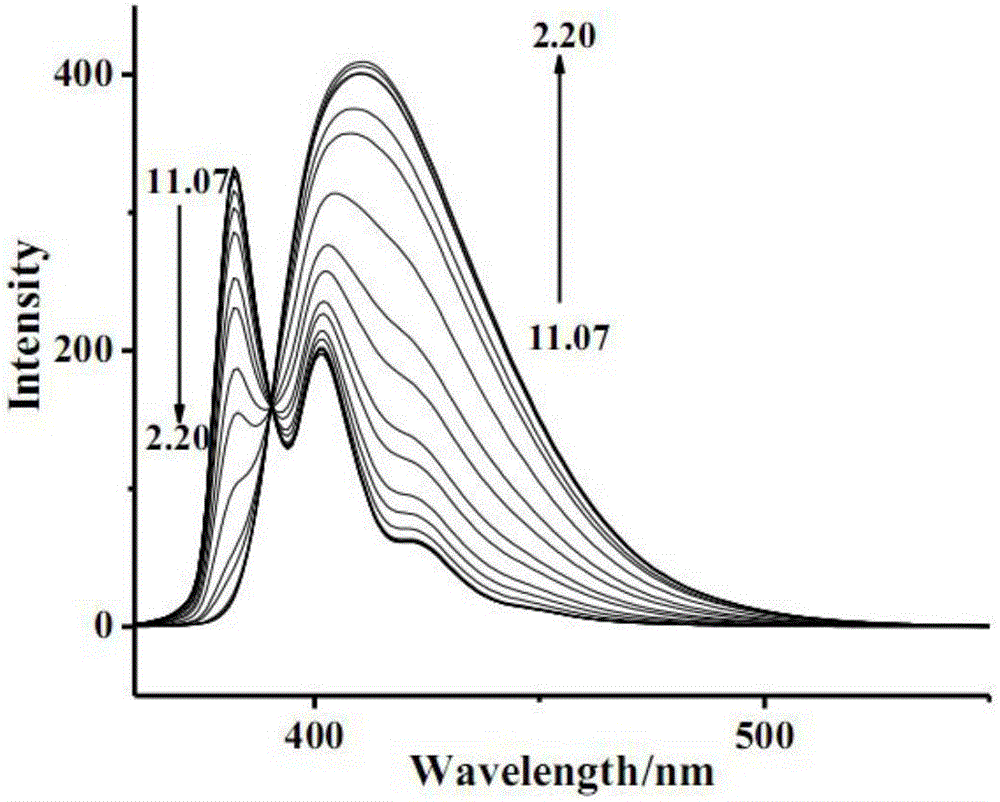
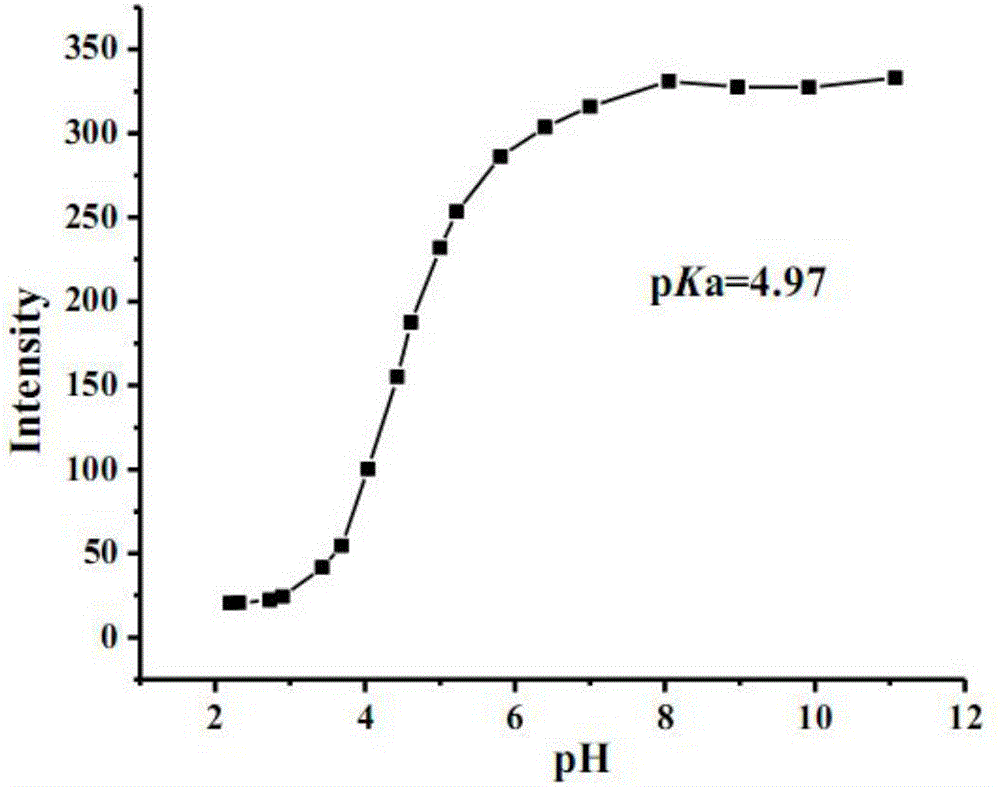
[0031] Prepare different pH values containing 1-pyrene formic acid (pH = 11.07, 9.92, 8.97, 8.05, 7.00, 6.40, 5.81, 5.22, 5.00, 4.61, 4.43, 4.04, 3.69, 3.43, 2.90, 2.73, 2.32, 2.20) Buffer solution (buffer solution concentration is 2.0×10 -3 mol / L, where the concentration of 1-pyrenecarboxylic acid is 2.0×10 -5 mol / L. Using 350nm as the excitation wavelength, measure and plot the fluorescence spectra of 1-pyrenecarboxylic acid in buffer solutions with different pH values (such as figure 1 Shown).
[0032] Such as figure 1 As shown, when excited with 350nm wavelength, the emission wavelength is the highest near 382nm and 401nm, and when the fluorescence emission wavelength of 1-pyreneformic acid is at 382nm, as the pH value decreases, 1-pyreneformic acid reacts to hydrogen ion The response of 1-pyreneformic acid shows fluorescence quenching; at 401nm, as the pH value decreases, the response of 1-pyrenec...
Embodiment 2
[0036] Selectivity of 1-pyrenecarboxylic acid fluorescent probe to hydrogen ion:
[0037] To a buffer solution with a pH of 5.0 (wherein the concentration of 1-pyrene formic acid is 2.0×10 -5 mol / L), dropwise containing Al 3+ Metal ion solution to make Al in the buffer solution 3+ The concentration of 1.0×10 -4 mol / L. Use the same method to configure each containing Ba 2 + , Cd 2+ , Cr 3+ , Co 2+ , Cu 2+ , Fe 3+ , Hg 2+ , Ni 2+ , Pb 2+ Buffer solution (the corresponding metal ion concentration in each buffer solution is 1.0×10 -4 mol / L, the concentration of 1-pyrenecarboxylic acid is 2.0×10 -5 mol / L).
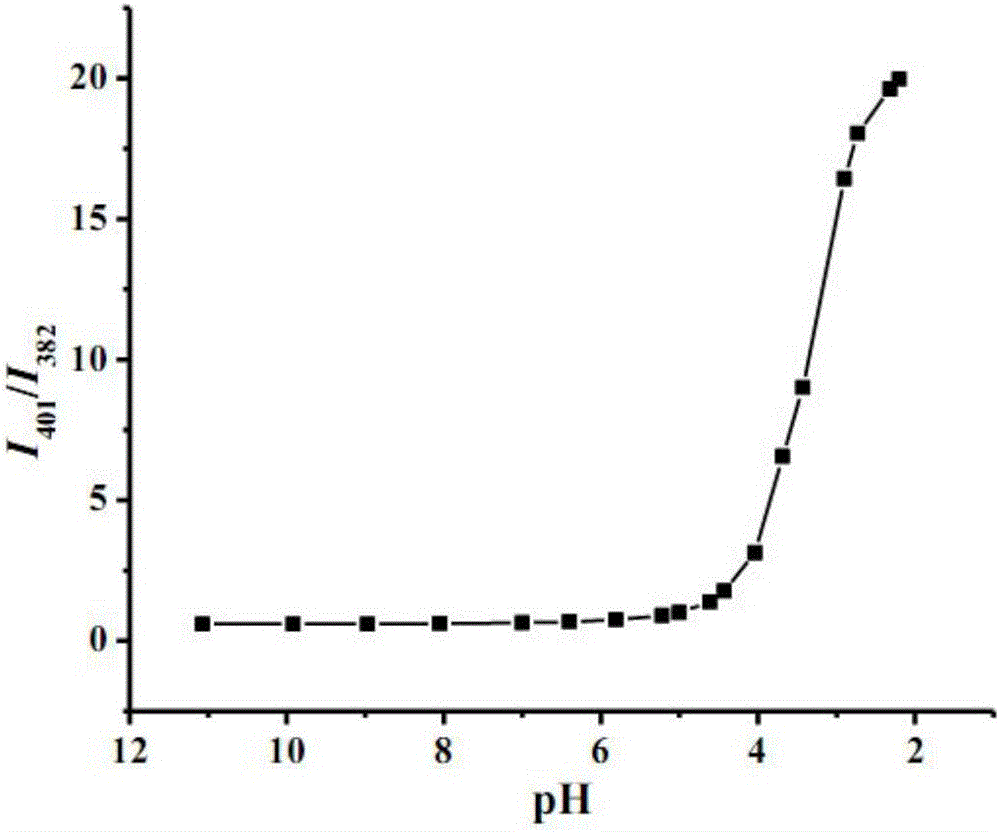
[0038] Using 350nm as the excitation wavelength, measure the fluorescence spectrum of each of the above-mentioned test solutions, and calculate I 401 / I 382 , In terms of fluorescence intensity ratio I 401 / I 382 Corresponding to different metal ions mapping (such as Figure 4 Shown).
[0039] Such as Figure 4 As shown, the fluorescence intensity ratio of 1-pyrenecarboxylic acid in e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com