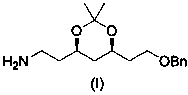
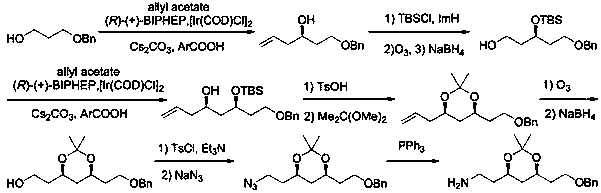
A kind of preparation method of atorvastatin chain measurement intermediate
A technology of reaction time and organic solvents, applied in the direction of organic chemistry, can solve the problems of unsuitable industrial production, limited industrial application, and inability to recycle, and achieve the effect of mild reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
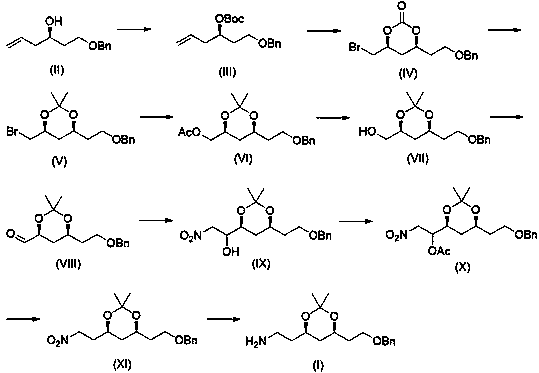
[0049] Embodiment 1, tert-butyl ( R )-1-benzyloxyhex-5-en-3-yl carbonate (III) preparation
[0050] Will( R )-1-benzyloxyhex-5-enol (II) (100g, 485mmol), dichloromethane (500mL), zinc acetate (9g, 4.9mmol) and di-tert-butyl dicarbonate (127g, 582mmol) In the reaction flask, heat and stir to reflux for 12 hours. After the reaction is complete, cool to room temperature and filter. After the filtrate is concentrated in solvent, it is distilled under reduced pressure to obtain light yellow oily liquid (III) (134 g, 91%).
Embodiment 2
[0051] Embodiment 2, tert-butyl ( R )-1-benzyloxyhex-5-en-3-yl carbonate (III) preparation
[0052] Will( R )-1-benzyloxyhex-5-enol (200g, 0.97mol), toluene (1L), zinc acetate (18g, 9.8mmol) and di-tert-butyl dicarbonate (233g, 1.07mol) in a reaction flask , heated and stirred at 80°C for 6h, after the reaction was completed, cooled to room temperature, filtered, and the filtrate concentrated the solvent and distilled under reduced pressure to obtain a light yellow oily liquid (III) (279g, 94%).
Embodiment 3
[0053] Embodiment 3, (4 S ,6 S )-4-(2-benzyloxyethyl)-6-bromomethyl-2-oxo-1,3-dioxane (IV) preparation
[0054] Compound (III) (50g, 163mmol), potassium carbonate (34g, 244mmol) and dichloromethane (500mL) were placed in a dry reaction flask, and bromine (31g, 196mmol) was added dropwise at -40°C with stirring. After dropping within 0.5h, keep stirring for 1h, after the reaction is completed, quench with 5% sodium bisulfite solution, separate layers, extract the water phase with dichloromethane, combine several layers, wash with water, dry over anhydrous sodium sulfate, and filter , the filtrate was concentrated to give colorless oily liquid (IV) (52.6g, 98%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com