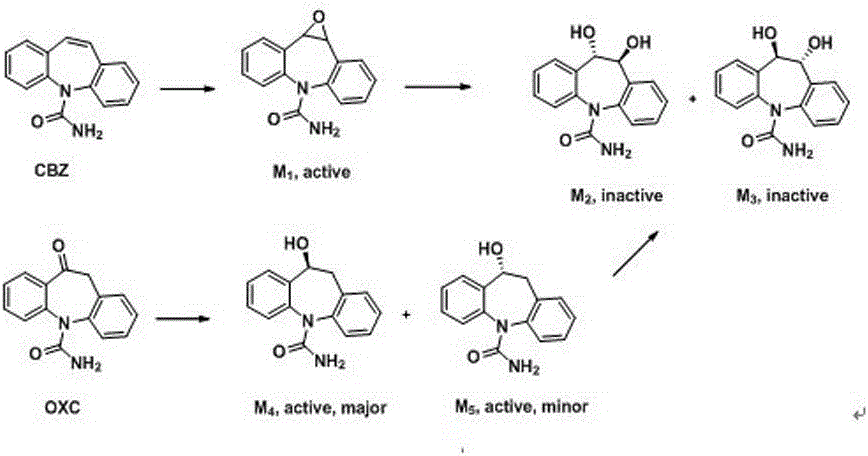
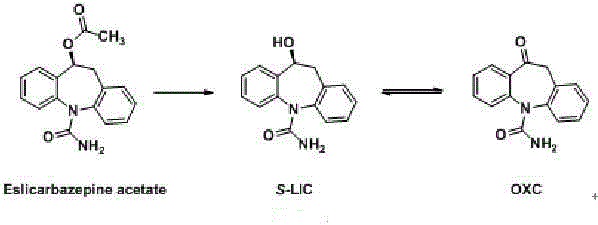
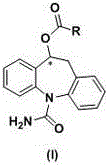
Brain-targeting eslicarbazepine ester prodrug and application thereof
An ester prodrug and prodrug technology, applied in the field of medicine, can solve problems such as adverse reactions and intolerance of patients, achieve high blood-brain barrier penetration, improve brain intake, and high brain targeting Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Synthesis of the compound of formula (I)
[0094] The synthetic route is as follows Figure 4 shown.
[0095] Taking the synthesis of compounds I-01 to I-16 as an example, the synthesis method will be further described.
[0096] At room temperature, add (100.0g, 0.4mol) oxcarbazepine, 400mL methanol and 200mL water into a 1L round-bottomed flask, add (15.0g, 0.4mol) sodium borohydride in batches, stir at room temperature for 30min and place at 45°C Continue to react for 1h. After the reaction was completed, the reaction solution was concentrated under reduced pressure to 200-300 mL, filtered, and washed with water three times to obtain 99.0 g of light yellow solid powder, which was compound 1, with a yield of 97.3%. m.p.188-192°C.
[0097]At room temperature, (9.2mmol) carboxylic acid, (18.4mmol) thionyl chloride and 1 drop of pyridine were added to 5mL of dichloromethane, and the reaction was refluxed for 3h. After the reaction was completed, the solvent and resid...
Embodiment 2
[0145] Preliminary Screening of Brain Uptake of Compounds of Formula (I) in Rats
[0146] Nine healthy Wistar rats weighed a certain amount of racemic licarbazepine, racemic licarbazepine acetate, I-03, I-04, I-07, I-11, I-13, I- 14. I-16, add 0.5% CMC-Na physiological saline (containing 0.9% NaCl) solution, and administer by intragastric administration at a dose of 0.13 mmol / kg. At 1, 5, 15, 30, 45, 60, and 180 minutes after administration, take 0.25 mL of whole blood from the venous plexus behind the eyes, put it in a heparinized EP tube containing the esterase inhibitor DDV, centrifuge at 3,500 rpm for 5 minutes at 4°C, and separate the plasma , take 0.1mL plasma, and store it at -80°C for testing.
[0147]27 healthy Wistar rats. Weigh a certain amount of racemic licarbazepine, racemic licarbazepine acetate, I-03, I-04, I-07, I-11, I-13, I-14, I-16, add 0.5% CMC-Na physiological saline (containing 0.9% NaCl) solution, administered by intragastric administration at a dose...
Embodiment 3
[0152] Further Verification of Brain Uptake of Compound of Formula (I) in Rats
[0153] In order to further verify that the compound of formula (I) has good brain uptake properties, 5 compounds of formula (I) with higher brain concentration were selected from Table 1 for chiral resolution to prepare the corresponding S-optical isomer The compound of formula (I) was evaluated for pharmacokinetics according to the method in Example 2, and compared with eslicarbazepine and eslicarbazepine acetate. The results show that, as shown in Table 2, compared with eslicarbazepine and eslicarbazepine acetate, the designed S-optical isomer formula (I) compound also has higher brain uptake, Especially compound I-26, namely (S)-10-(3-thiophene) acyloxy-10,11-dihydro-5H-dibenzo[b,f]azepine-5-carboxamide, which enters After the brain is rapidly converted to eslicarbazepine, the brain content is as high as 501ng / g, which is 3.1 times that of eslicarbazepine alone and 2.2 times that of eslicarbaz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com