Methods and compounds for the synthesis of fused aminodihydrothiazine derivatives
A technology for compounds and mixtures, applied in organic chemistry, drug combinations, neurological diseases, etc., which can solve problems such as undeveloped
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
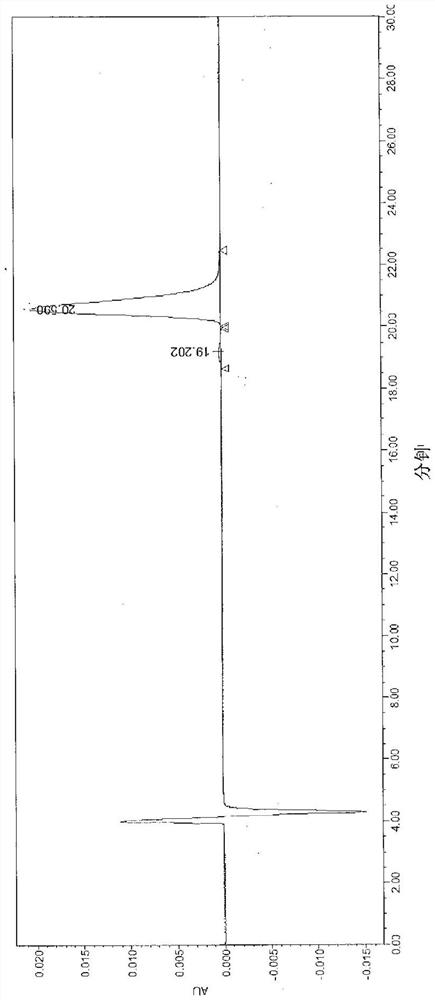
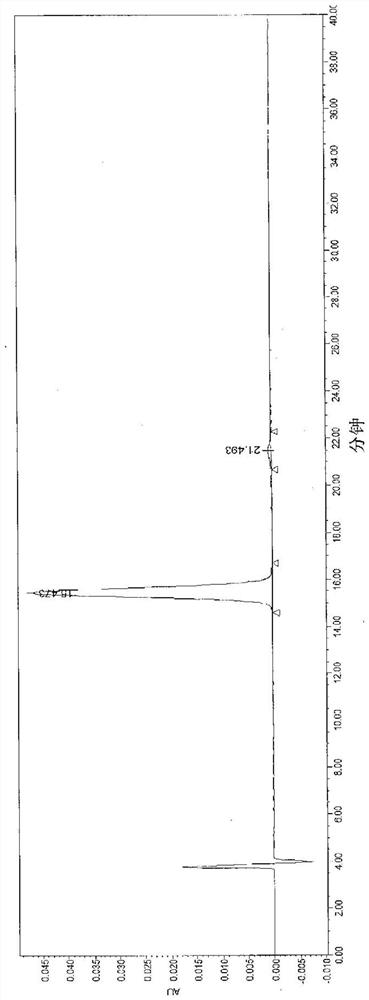
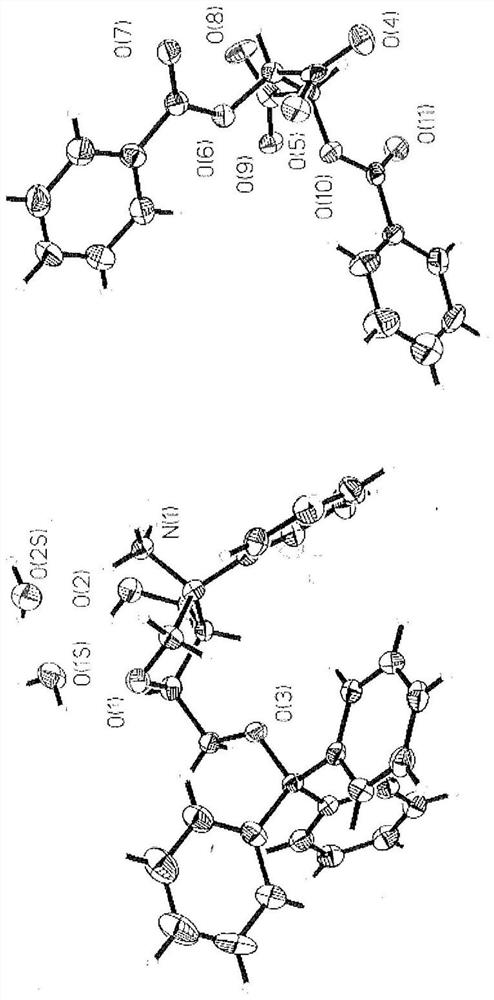
Image
Examples
Embodiment 1
[0388] Example 1 : Preparation of 6a-(2-fluorophenyl)-4-(trifluoromethyl)hexahydrofuro-[3,4-c]isoxazole
[0389]
[0390] tert-butyl 2-(1,1,1-trifluorobut-3-en-2-yloxy)acetate at rt under nitrogen The reaction vessel was charged with toluene (3.2 L), THF (0.60 L) and propylene Aldehyde (0.40 L, 5.985 mol). (Trifluoromethyl)trimethylsilane (1.003 kg, 7.059 mol) was added at 17°C. The reaction mixture was cooled to 2.5 °C and TBAF (0.01 M in THF, 0.400 L, 0.004 mol) was added over 2 hours. During the addition of TBAF, the temperature of the reaction mixture was raised to 65°C. The reaction mixture was cooled to 0 °C and after 2 h, tetra-n-butylammonium bisulfate (0.171 kg, 0.503 mol) was added followed by tert-butyl bromoacetate (0.987 kg, 5.064 mol). Sodium hydroxide (50% wt in water, 4.2 kg, 52.6 mol) was added over 2 h while maintaining the temperature below 10 °C. After 2 h at 0-5 °C, water (2.9 L) and methyl tert-butyl ether (6.0 L) were added to the reaction mixture...
Embodiment 2
[0414] Example 2 : Preparation of 6a-(2-fluorophenyl)-4-methylhexahydrofuro[3,4-c]isoxazole
[0415]
[0416] tert-Butyl 2-(but-3-en-2-yloxy)acetate: The reactor was charged with tetrabutylammonium bisulfate (0.17 Wt, 0.10 eq) and toluene (2.6 Wt, 3.0 V). The mixture was stirred and cooled to 0-5 °C. While maintaining the internal temperature below 10°C, add 50wt% sodium hydroxide aqueous solution (4.5wt, 3.0V, 10.5 equivalents; 50%wt sodium hydroxide prepared from 2.25wt of sodium hydroxide and 2.25wt of water), followed by 3-buten-2-ol (0.45Wt, 0.53V, 1.20 equiv). The mixture was stirred at 0-10°C for 15 minutes. Tert-butyl bromoacetate (1.0 Wt, 1.00 equiv) was added while maintaining the internal temperature at 0-10 °C. After the addition, the mixture was stirred at 0-5°C for 1 h and monitored for complete consumption of tert-butyl bromoacetate (target >98% conversion). The reactor was charged with water (3.0Wt, 3.0V) and MTBE (4.4Wt, 6.00V) and raised to 20-25°C. ...
Embodiment 3
[0453] Example 3 : Preparation of 6a-(2,3-difluorophenyl)-4-((trityloxy)methyl)hexahydrofuro[3,4-c]isoxazole
[0454]
[0455] 1-morpholino-2-(1-(trityloxy)but-3-en-2-yloxy)ethanone. To the reactor with 1-(trityloxy)but-3-en-2-ol 13 (41.6 g, 0.111 mol, 1.0 eq) was added toluene (146 mL). The resulting solution was cooled to 0-5°C and tetra-n-butylammonium bisulfate (7.52 g, 0.0222 mol, 0.20 equiv) was added. 4-(Chloroacetyl)morpholine (18.1 g, 0.111 mol, 1.00 equiv) was added at 0-5°C. Sodium hydroxide (50% wt. in water; 88.6 g, 1.10 mol, 10 equiv) was cooled to 15°C and added to the reaction mixture while T 99%). 2-Methoxy-2-methylpropane (146 mL) and water (146 mL) were added to the reaction mixture while T<20°C. The organics were washed with 18% aqueous NaCl (73 mL) and saturated aqueous NH4Cl (21 mL). The organics were filtered through celite (10.4 g) to remove particulates and rinsed with 2-methoxy-2-methylpropane (83 mL). The solvent was evaporated under vacuum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com