Polymolecular marker composition used for lung cancer diagnosis
A protein marker and lung cancer technology, applied in the field of disease diagnosis, can solve the problems of increased difficulty in lung cancer diagnosis and screening, increased difficulty of diagnosis, and low early cancer diagnosis rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
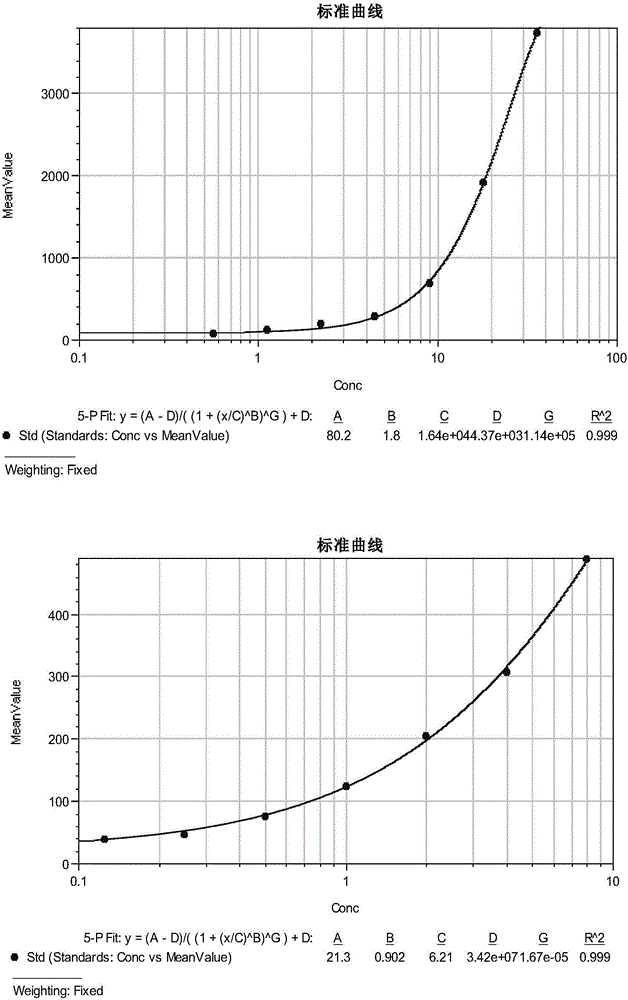
[0084] (1) Preparation of liquid phase chip (each protein is coated with corresponding microspheres, the preparation method is the same)
[0085] 1) Take the microsphere suspension (magnetic microspheres, bio-rad), vortex and sonicate for about 20s respectively, take 200ul (about 2.5×10 6 microspheres) in a clean centrifuge tube (USA, Axygen), marked with the microsphere model and protein name;
[0086] 2) 14000g, room temperature (about 25°C), 4min; carefully suck off the supernatant, and a small amount of supernatant can be left to reduce loss;
[0087] 3) Add 100ul ddH 2 O, vortex and sonicate for about 20s respectively;
[0088] 4) 14000g, room temperature, 4min; carefully suck off the supernatant, in order to reduce the loss, a small amount of supernatant can be left, add 160ul pH6.2, 0.1M NaH 2 PO 4 , respectively vortex and sonicate for about 20s;
[0089] 5) Quickly add 20ul S-NHS, vortex and mix;
[0090] 6) Quickly add 20ul EDC, vortex and sonicate for about 20...
Embodiment 1
[0138] Example 1, screening of markers
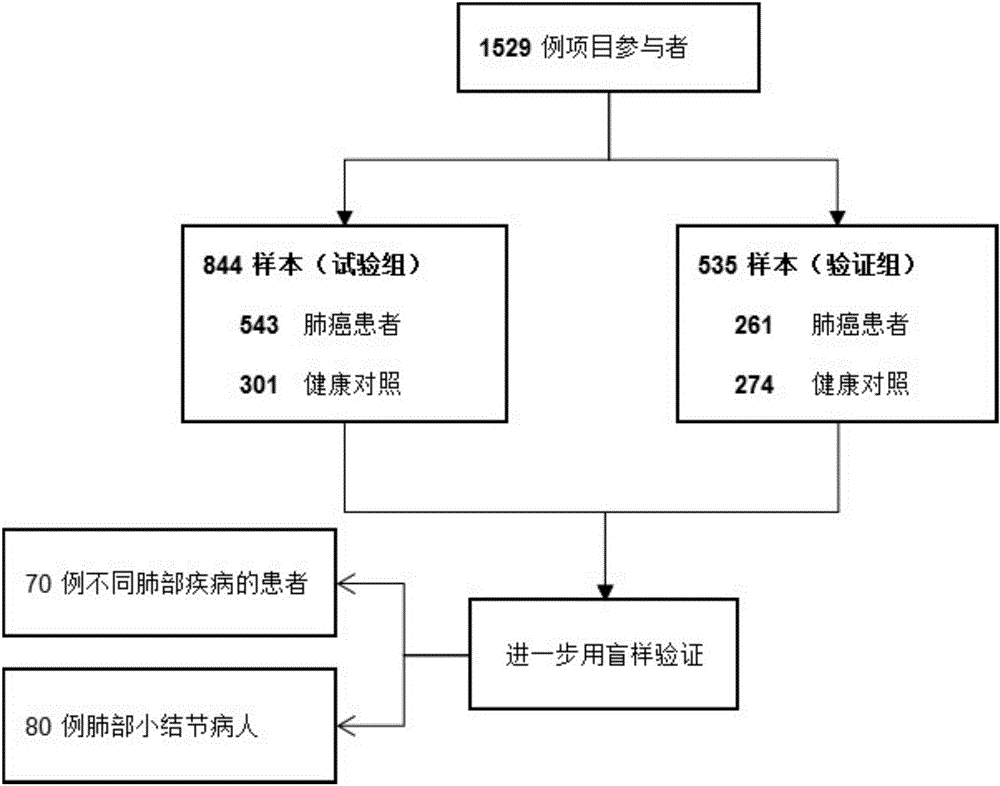
[0139] The inventor collected 804 cases of patients in total, including 543 cases in the test group and 261 cases in the verification group. There were 575 cases in the healthy group, including 301 cases in the test group and 274 cases in the verification group. In addition, 70 patients with other benign lung diseases and 80 patients with small pulmonary nodules were included.
[0140] Statistical results show that there is no significant statistical difference in gender, smoking status, age and other factors between the healthy group and the experimental case (test group, verification group) group.
[0141] The clinical case characteristics of the subjects are summarized in Table 3.
[0142] table 3
[0143]
[0144] P value χ 2 test.
Embodiment 2
[0145] Example 2. Screening and validation of lung cancer-related molecular markers
[0146] In the present invention, the screening process of lung cancer markers is as follows: figure 1 .
[0147] The purpose of this example is to screen out molecular markers that can distinguish healthy people from lung cancer patients. A large-scale comparison of proteins expressed in serum samples of lung cancer patients with those of healthy individuals is expected to screen out lung cancer-related molecular markers.
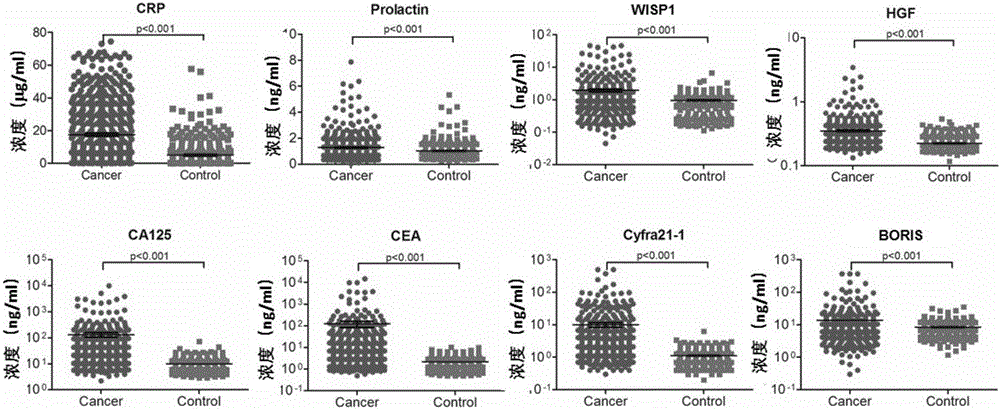
[0148] After a large sample size analysis and screening, as a result, the inventors screened out a total of 8 proteins, which are highly expressed in lung cancer patients, and can better distinguish healthy people from lung cancer patients, including: C-reactive protein (CRP), prolactin Prolactin, hepatocyte growth factor (HGF), CEA, WIPS1, Cyfra21-1, CA125, BORIS (P image 3 .
[0149] Therefore, the present inventors found 8 effective molecular markers in total.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com