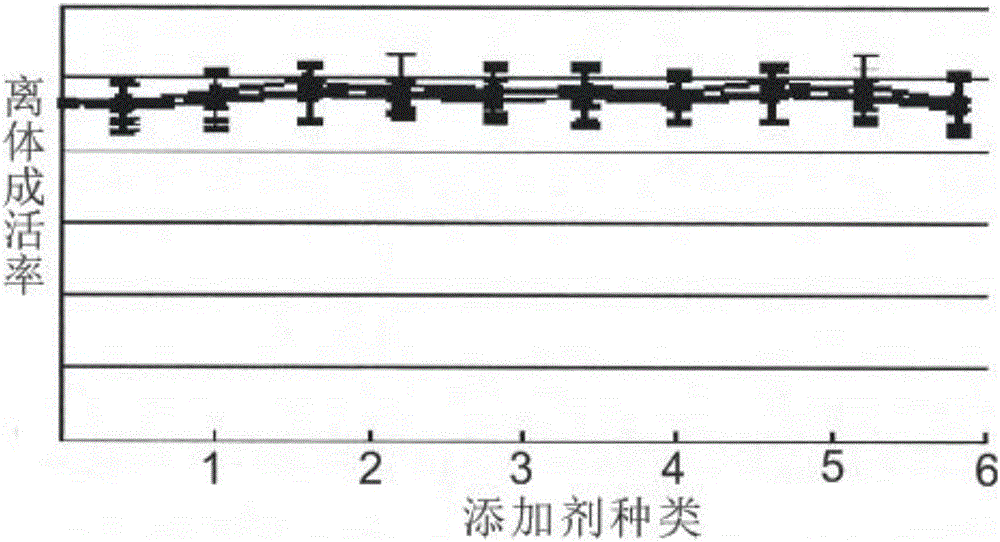
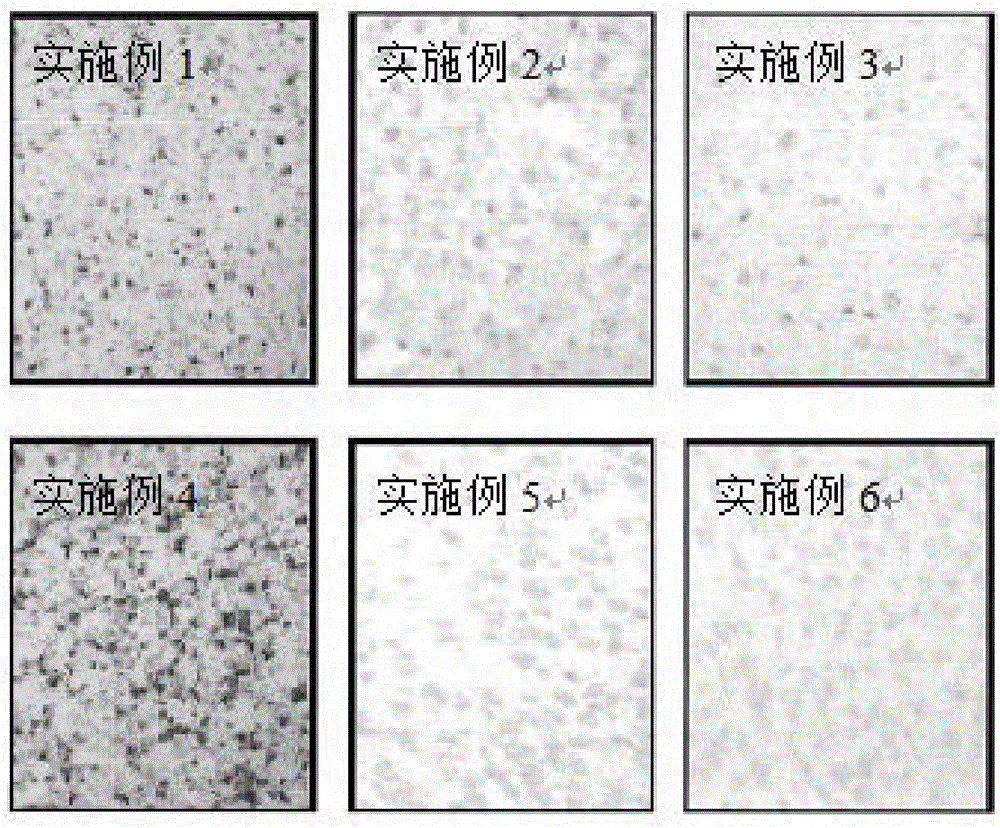
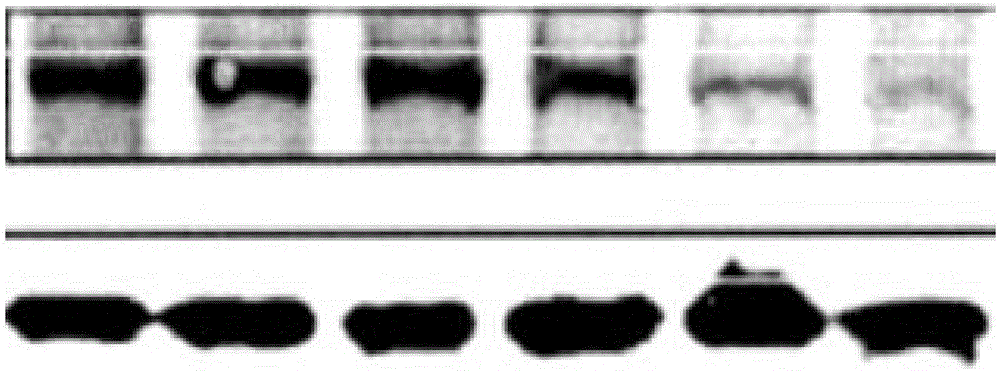
Resurrection hormone composition capable of improving filling fat survival rate and reducing fat fibration and calcification
A survival rate and filling technology, applied in the field of bioengineering, can solve problems such as difficulty in dispensing lipoactivin, difficult cost and technical conditions, and complex life characteristics, so as to reduce the possibility and severity of rejection and improve Effects on Survival, Likelihood and Severity Reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A resurrection formula that can improve the survival rate of filling fat and reduce fat fibrosis and calcification is composed of the following raw materials in parts by weight:
[0023] 20 parts of mother cells from the recipient type, 20 parts of glucose base solution, 10 parts of recombinant human interleukin-II, 12 parts of cytokines, 1 part of human pH regulator, 5 parts of amino acids, human acidic fibroblast factor rh- 6 parts of aFGF, 4 parts of cell growth stimulating activity GRO / MGSA, 0.5 part of lysozyme.
[0024] According to the above technical solution, the mother cells obtained from the recipient type have cell functional structures such as organelles removed, and only the cell fluid is retained.
[0025] According to the above technical solution, the concentration of the glucose base solution is 0.100 mol / L.
[0026] According to the above technical solution, the recombinant human interleukin-II includes any three or four of α1b, α2a, ξ, ω, μ, τ, and κ...
Embodiment 2
[0031] A resurrection formula that can improve the survival rate of filling fat and reduce fat fibrosis and calcification is composed of the following raw materials in parts by weight:
[0032] 23 parts of mother cells from the recipient type, 22 parts of glucose base solution, 12 parts of recombinant human interleukin-II, 13 parts of cytokines, 1.5 parts of human pH regulator, 6.5 parts of amino acids, human acidic fibroblast factor rh- 8 parts of aFGF, 6 parts of cell growth stimulating activity GRO / MGSA, 0.75 parts of lytic enzyme.
[0033] According to the above technical solution, the mother cells obtained from the recipient type have cell functional structures such as organelles removed, and only the cell fluid is retained.
[0034] According to the above technical solution, the concentration of the glucose base solution is 0.180 mol / L.
[0035] According to the above technical solution, the recombinant human interleukin-II includes any three or four of α1b, α2a, ξ, ω, ...
Embodiment 3
[0040] A resurrection formula that can improve the survival rate of filling fat and reduce fat fibrosis and calcification is composed of the following raw materials in parts by weight:
[0041] 26 parts of mother cells from the recipient type, 24 parts of glucose base solution, 14 parts of recombinant human interleukin-II, 14 parts of cytokines, 2 parts of human pH regulator, 8 parts of amino acids, human acidic fibroblast factor rh- 9 parts of aFGF, 8 parts of cell growth stimulating activity GRO / MGSA, 1 part of lysozyme.
[0042] According to the above technical solution, the mother cells obtained from the recipient type have cell functional structures such as organelles removed, and only the cell fluid is retained.
[0043] According to the above technical solution, the concentration of the glucose base solution is 0.260 mol / L.
[0044] According to the above technical solution, the recombinant human interleukin-II includes any three or four of α1b, α2a, ξ, ω, μ, τ, and κ1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com