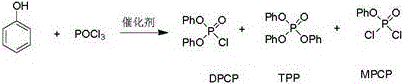
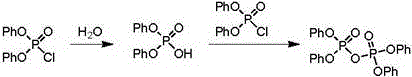Preparation method of diphenyl phosphoryl chloride
A technology of diphenyl chlorophosphate and phosphorus oxychloride, which is applied in the chemical industry, can solve problems such as unknown yield, harm to human body and environment, and damage to aluminum trichloride, and achieve high yield and increase single-batch production capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] Below in conjunction with embodiment the present invention is described in further detail.
[0015] Add 178 grams (1.9 moles) of phenol, 153 grams (1 mole) of phosphorus oxychloride, 1.8 grams of triphenylphosphine and 2.7 grams of triphenylphosphine oxide in example 1, 500 milliliters of four-neck flasks, heat up and stir the reaction, through 3 Heating up to 140°C per hour, heat preservation reaction, the phenol content in the GC is less than 0.5%, the reaction liquid is rectified in the rectification tower, and 216 grams of distillate at 150±2°C / 4mmHg is collected, which is the finished diphenyl chlorophosphate, with a yield of 84.8% , GC purity 99.4%. Distillation bottoms containing triphenylphosphine and triphenylphosphine oxide can be applied mechanically to the next batch of reactions.
[0016] Example 2, 178 grams (1.9 moles) of phenol, 153 grams (1 mole) of phosphorus oxychloride and the rectification base material of Example 1 were added to a 500 milliliter f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com