Diarylheptanes
A diaryl heptane and compound technology, applied in the field of diaryl heptane compounds, can solve problems such as hindering cellular immune response and large side effects, and achieve the treatment of Alzheimer's disease, treatment of inflammation, and inhibition of nitric oxide generated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
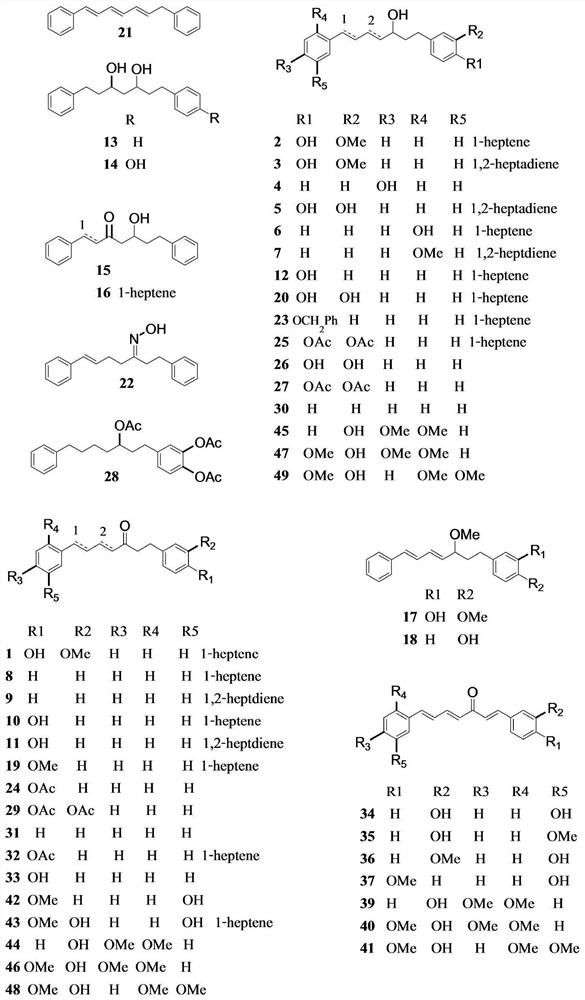
[0045] Example 1 Extraction, separation and identification of diaryl heptane compounds
[0046] 1.1 Instruments and materials
[0047] Perkin-Elmer FT-IR infrared spectrophotometer, KBr pellets; nuclear magnetic resonance experiments using BrukerAVANCE 400 nuclear magnetic resonance; using ES-TOFMS and ES-MS mass spectrometry using Bruker microTOF and aFinnigan LC-Q II mass spectrometer; JASCO-1020 polarimeter; high performance liquid chromatography: Agilent 1260HPLC, the preparative column uses Kromasil 100-10-C18; Merck silica gel 60 (fine than 0.063 mm) and cross-linked dextran LH-20 from Pharmacia. The rhizomes of Curcuma comosa are in Nakhon Pathom, Sakon Nakhon and Prachin Buri, Thailand, and voucher specimens (Apichart Suksamrarn, Nos. 052 and 074) are deposited at the Faculty of Science, Ramkhamhaeng University.
[0048] 1.2 Separation and extraction of compounds
[0049] The rhizome (5.2kg) of C.comosa was sliced, air-dried, ground and sequentially soaked with n-hex...
Embodiment 2
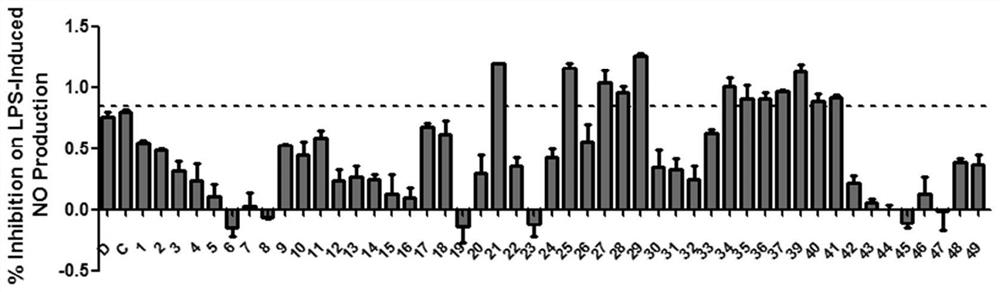
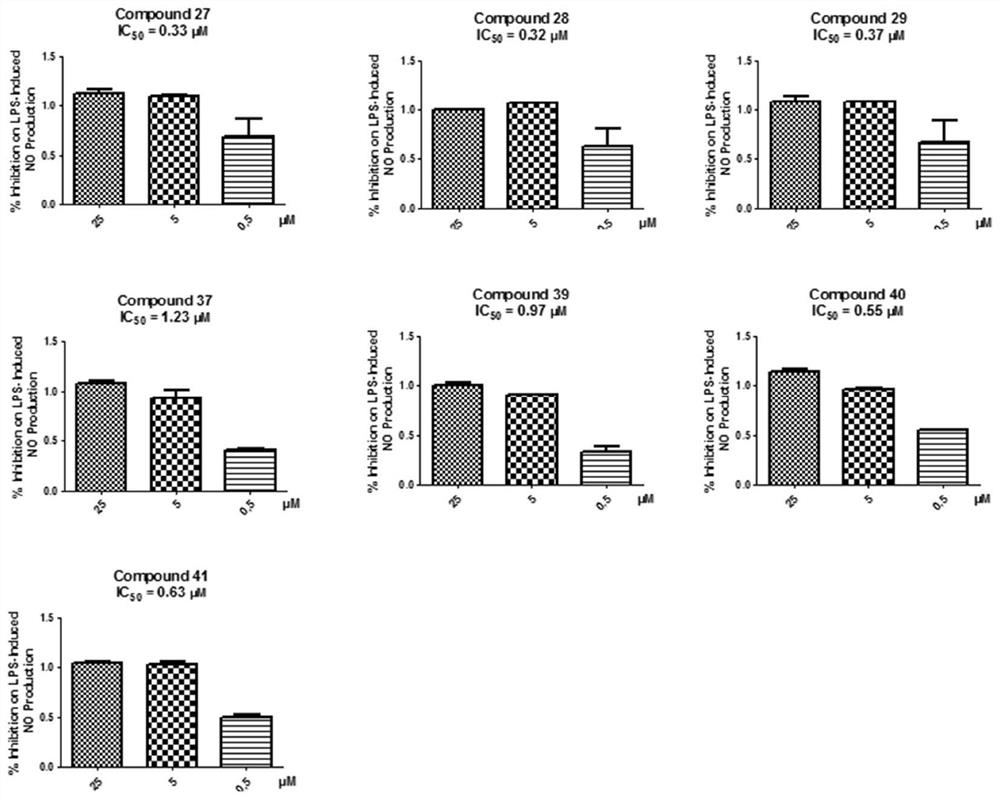
[0156] Example 2 The activity of diarylheptane compounds in inhibiting NO production.
[0157] 1 material
[0158] Compounds (1-37, 39-49); mouse RAW264.7 macrophages; endotoxin (LPS, 0.5 μg / mL); BAY (10 μM, positive control) NO detection kit (Beyotime, Haimen, China).
[0159] 2 main instruments
[0160] Fluorescent microplate reader (Thermo Scientific, Waltham, MA, USA)
[0161]3 methods
[0162] 3.1 Determination of NO production by LPS-stimulated RAW264.7 macrophages
[0163] RAW264.7 macrophages were seeded in 96-well plates, and the seeding volume per well was 1×10 4 After the cells were inoculated and adhered to the wall, the compounds to be tested (1-37, 39-49) were added to incubate for 2 h, and then LPS (final concentration: 0.5 μg / mL) was added to continue culturing for 24 h. Finally, take the 96-well plate culture medium and use the NO kit to detect the NO content by measuring its OD 550 To represent. Three replicate wells were taken for each experiment, and...
Embodiment 3 2
[0173] Example 3 Inhibitory effect of diarylheptane compounds on macrophage proliferation
[0174] 1 material
[0175] Compounds (1-37, 39-49); mouse RAW264.7 macrophages; Alamar-Blue reagent.
[0176] 2 main instruments
[0177] Fluorescent microplate reader (Thermo Scientific, Waltham, MA, USA)
[0178] 3 methods
[0179] 3.1 Effects of compounds (1-37, 39-49) on the proliferation of RAW264.7 macrophages
[0180] Inoculate RAW264.7 macrophages in 96-well plate, inoculate 1×10 per well 4 After the cells are inoculated and attached to the wall, the compounds to be tested (1-37, 39-49) are added and incubated for 24 hours. Then add Am-Blue cell proliferation and activity detection reagent (SunBio TM ) 10 μL and incubate in the incubator for 2-6 hours. When the color of the culture medium changed from indigo blue to pink, the relative fluorescence unit (RFU value) of each well was measured with a fluorescent microplate reader, the excitation light wavelength was 560nm, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com