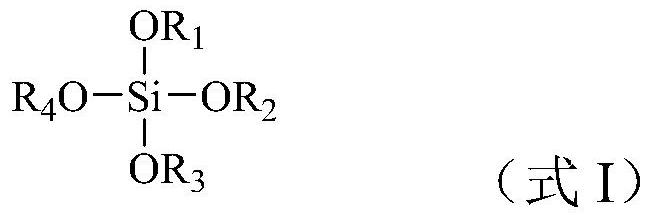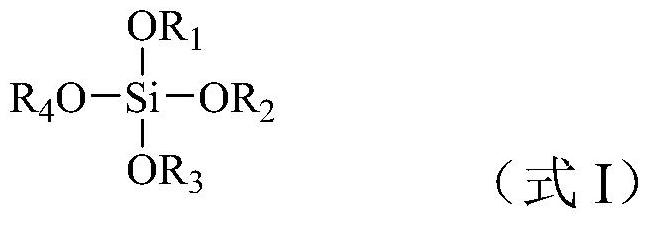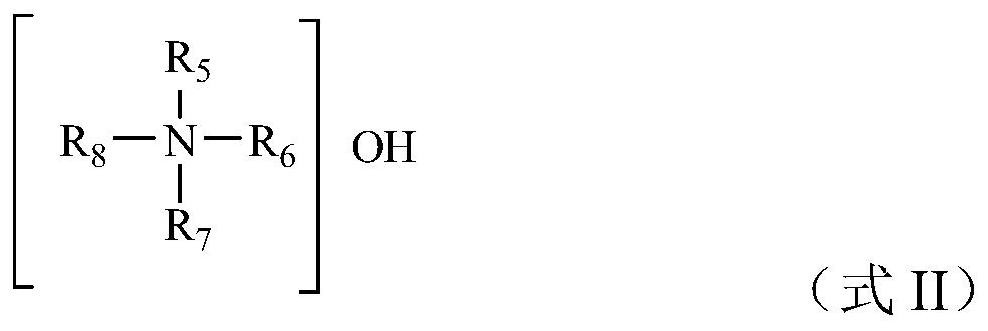Noble metal-modified mesoporous titanium-silicon molecular sieve, synthesis method and application thereof, and a method for oxidizing cyclic ketones
A technology of titanium silicon molecular sieve and oxidizing cyclic ketone, which is applied in the direction of molecular sieve catalyst, molecular sieve and alkali exchange compound, non-metallic element, etc., to achieve the effect of reducing dosage, high crystallinity and improving synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0021] According to a preferred embodiment of the present invention, the method of the present invention comprises:
[0022] (1) Hydrolyze the organosilicon source, titanium source, precious metal source, alkali source and surfactant to obtain a mixture A, perform the first crystallization on the obtained mixture A, and dry or not dry the solid product after the first crystallization After roasting;
[0023] (2) In the presence of water, the calcined product is mixed with an inorganic silicon source to obtain a mixture B, and the obtained mixture B is subjected to a second crystallization.
[0024] According to the method of the present invention, preferably in step (2), the molar ratio of the inorganic silicon source in terms of silicon oxide to water is 1:(5-250). Wherein, the water in step (2) can be derived from raw materials such as inorganic silicon sources, or can be additionally added, as long as the requirements of the present invention are met.
[0025] According t...
Embodiment 1
[0100] First, 50 grams of tetraethyl orthosilicate and cetyltrimethylammonium bromide were added to the tetrapropylammonium hydroxide aqueous solution in which tetrabutyl titanate and palladium chloride were pre-dispersed and stirred, and the orthosilicate The molar ratio of tetraethyl titanate, tetrabutyl titanate, palladium chloride, tetrapropylammonium hydroxide, cetyltrimethylammonium bromide and water is 1:0.02:0.01:0.45:0.15:85, Among them, organosilicates are represented by SiO 2 Meter, titanium source as TiO 2 Alkali source is measured in N. After the silicon ester is hydrolyzed (the hydrolysis rate of the organic silicon source is 100%), the mixture is transferred to a sealed reactor for hydrothermal crystallization at 110°C for 12 hours. After cooling, the reactor is opened to the crystallization system Add silica gel A and mix well, wherein the molar ratio of silica gel to organic silicon source is 1:0.2 in terms of silica, and then continue to crystallize the mixt...
Embodiment 2
[0102] First, 50 grams of tetramethyl orthosilicate and surfactant tetradecyltrimethylammonium bromide are added to the tetrapropylammonium hydroxide aqueous solution in which tetraethyl titanate and palladium acetate have been dispersed in advance and stirred and mixed, wherein The molar ratio of tetramethyl orthosilicate, tetraethyl titanate, palladium acetate, tetrapropylammonium hydroxide, surfactant and water is 1:0.008:0.02:0.15:0.05:25, in which organic silicate is SiO 2 Meter, titanium source as TiO 2 Alkali source is calculated in N. After the silicon ester is hydrolyzed (the hydrolysis rate of the organic silicon source is 50%), the mixture is transferred to a sealed reactor for hydrothermal crystallization at 120°C for 5 hours. After cooling, the reactor is opened to the crystallization system Add silica gel B and mix well, wherein the molar ratio of silica gel to organic silicon source is 1:0.1 in terms of silica, and then continue to crystallize the mixture in a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com