fgf1 mutant and its medical application
A technology of mutants and drugs, applied in the field of protein, can solve the problems of induced hyperplasia and achieve the effect of convenient industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 FGF1 of the present invention △HS and its in vitro activity
[0029] Cloning, expression and purification of FGF1 by conventional means △HS . Briefly, the full-length human wild-type FGF1 (abbreviated as FGF1 WT , positions 1-155) were cloned into the pET30a expression vector, and then the three mutations Lys127Asp, Lys128Gln and Lys133Val were introduced using the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The expression vector with the mutant was transformed into Escherichia coli BL21(DE3), cultivated at 37°C, when A 600 When reaching 0.5, add 1 mM IPTG to continue culturing for 4 hours. After lysing, the cells were purified by cation-exchange chromatography and gel-exclusion chromatography (GE Healthcare, Piscataway, NJ) to obtain FGF1 with a purity >98%. △HS . Other control proteins can also be prepared in a similar manner.
[0030] Detected by SPR biosensor chip (BIAcore 2000 system (GE Healthcare, Piscataway, NJ)), com...
Embodiment 2
[0034] Example 2 FGF1 of the present invention △HS in vivo activity studies
[0035] (1) Normal mice
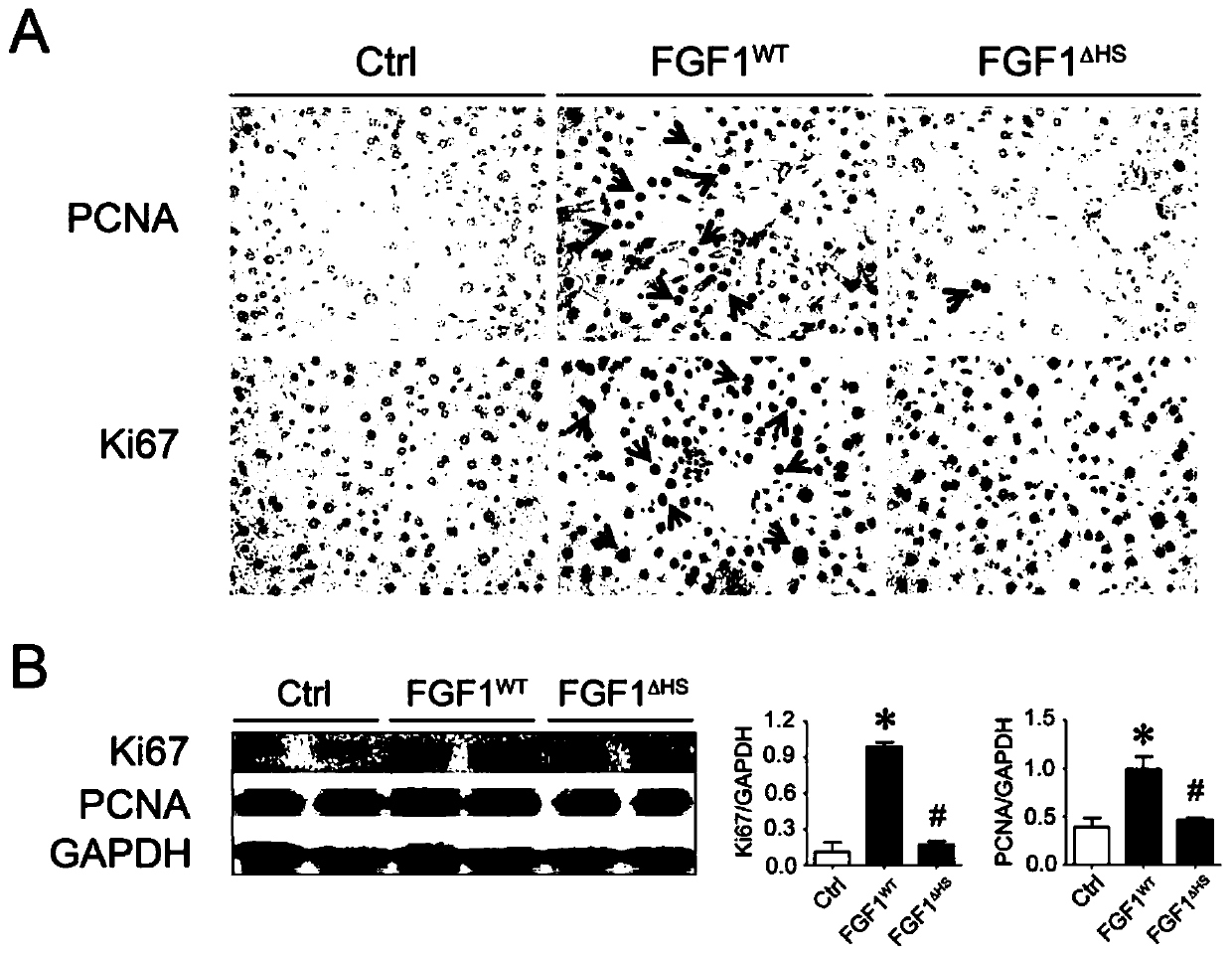
[0036] everyday to normal C57BL / 6J Mice administered FGF1 WT and FGF1 △HS (0.5 mg / kg body weight), for 3 months, with PCNA and Ki67 ( figure 2 A) Immunohistochemical staining and Western blotting ( figure 2 B) Mouse livers were analyzed for hyperplasia. FGF1 WT was observed to cause hyperplasia in mice, while FGF1 △HS Hyperplasia did not appear relative to the PBS control group.
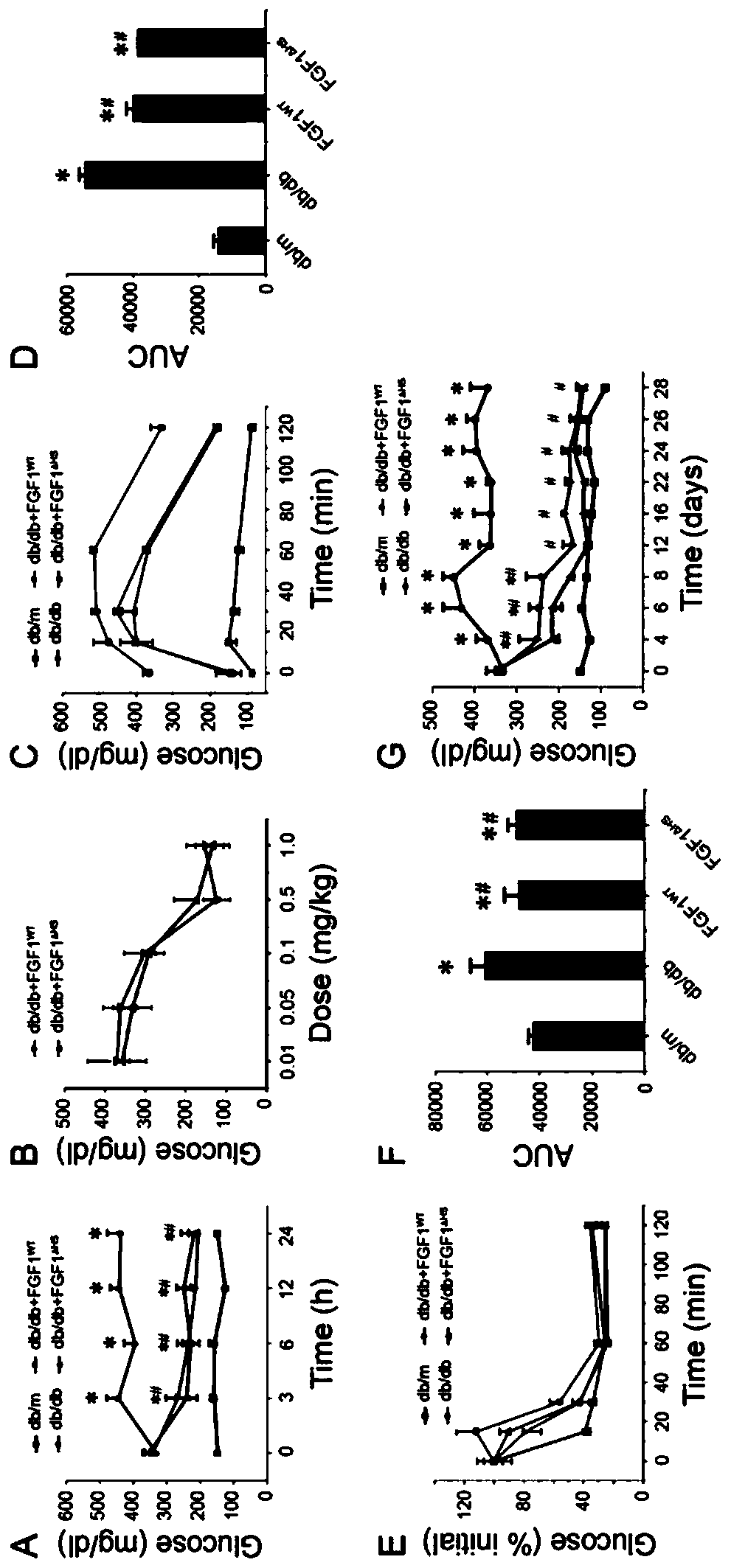
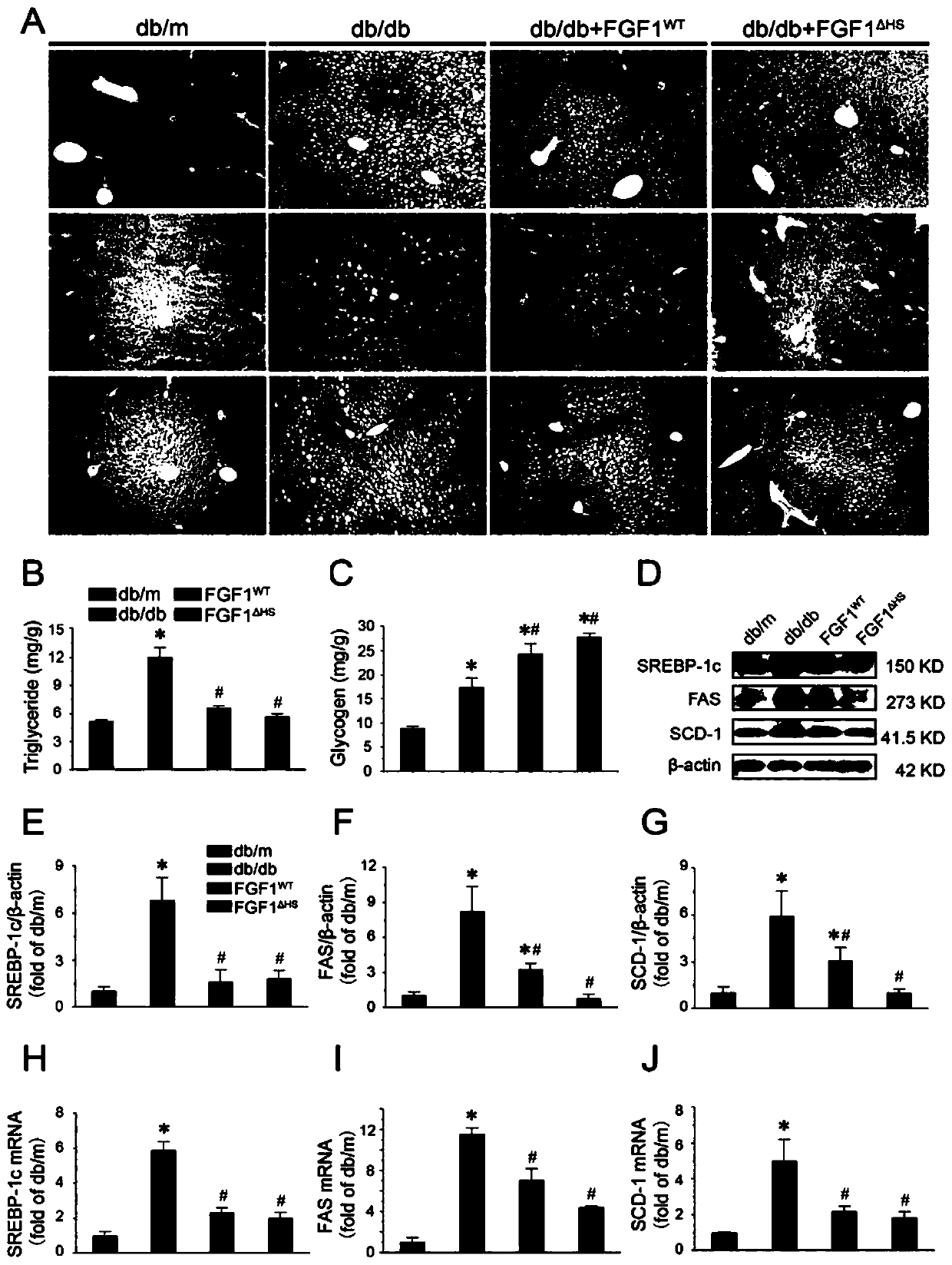
[0037] (2) FGF1 WT and FGF1 △HS right db / db Effects on Blood Glucose Levels and Insulin Sensitivity in Mice
[0038] Diabetic model ( db / db ) mice (C57BLKS / J- lepr db / lepr db ) and their control phenotypically normal mice ( db / m ) were purchased from the Model Animal Research Center of Nanjing University.
[0039] exist db / db In mice, rapid injection of FGF1 WT and FGF1 △HS (0.5 mg / kg body weight) can significantly reduce blood sugar levels, and the effect can basical...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com