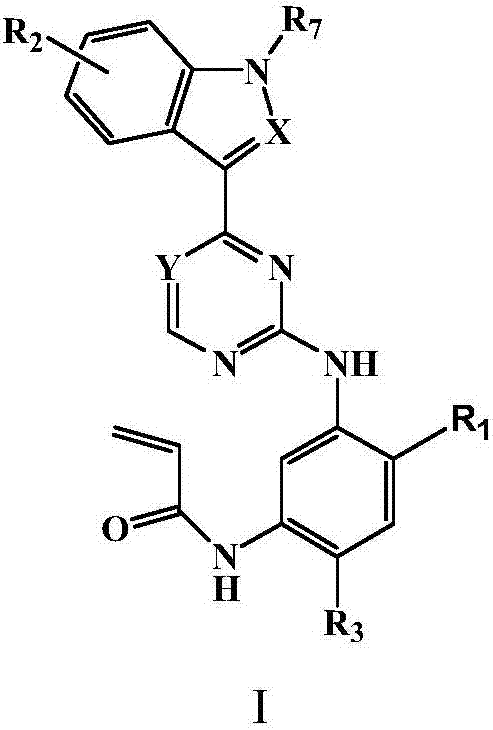
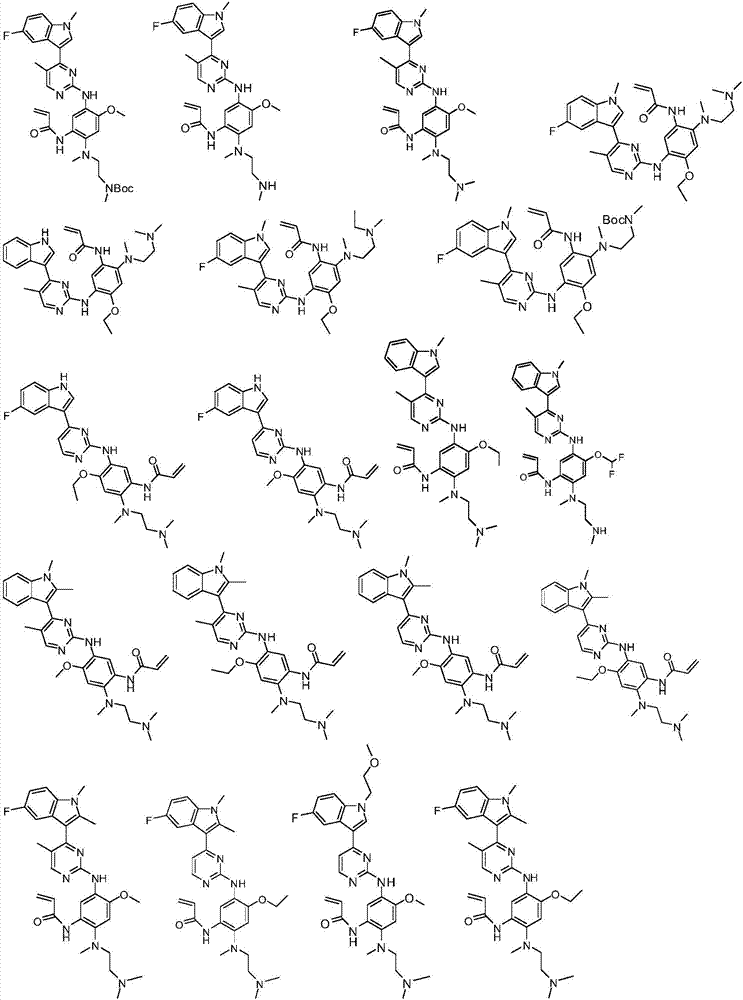
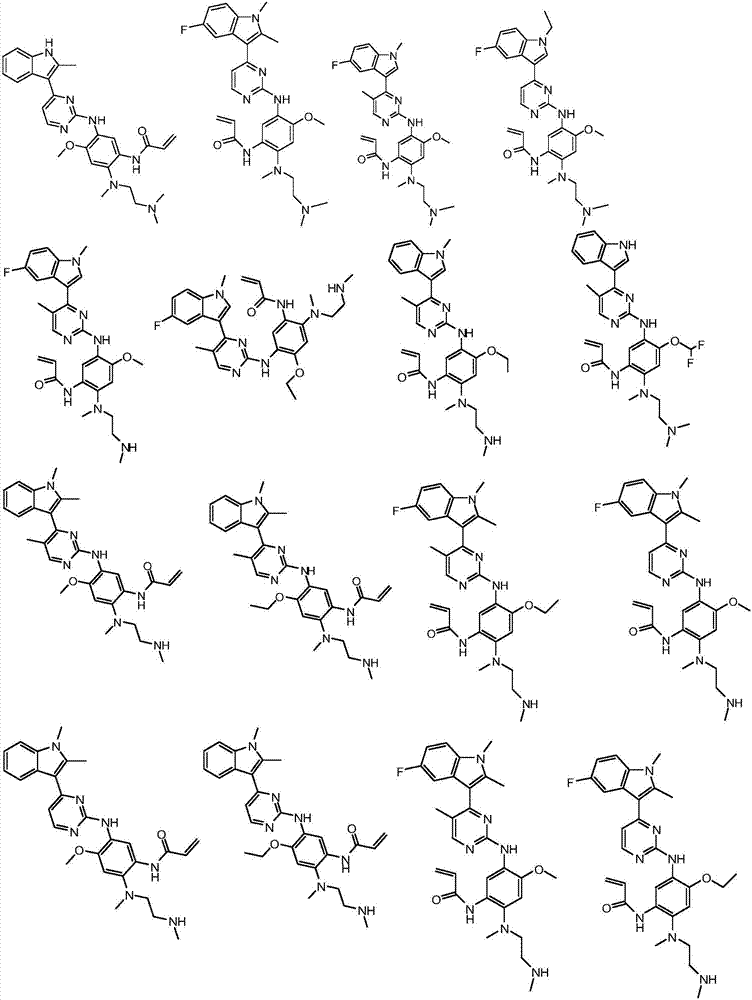
Substituted indole or indole pyrimidine derivative as well as preparation method and application of substituted indole or indole pyrimidine derivative
A compound and selected technology can be used in drug combinations, pharmaceutical formulations, medical preparations containing active ingredients, etc., which can solve the problem of limiting clinical drug dosage, failing to reach the effective exposure of drug-resistant tumors, and showing no obvious drug-resistant tumors. issues of efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1: 2-((2-acrylamido-4-(4-(5-fluoro-1-methyl-1H-indol-3-yl)-5-methylpyrimidin-2-ylamine Synthesis of 5-methoxyphenyl)(methyl)amino)ethyl(methyl)carbamate tert-butyl (compound 1):
[0086]
[0087] a. Synthesis of 3-(2-chloro-5-methylpyrimidin-4-yl)-5-fluoro-1-methyl-1H-indole
[0088]
[0089] The raw material 5-fluoroindole (10g, 1eq) was added in a round bottom flask, 150ml of DMF was added, the temperature was cooled to about -10 degrees Celsius and 60% sodium hydride (6g, 2eq) was added, and after stirring for about 20 minutes, methyl iodide ( 15.7g, 1.5eq), the temperature was raised to room temperature and the reaction was stirred. After the reaction was detected by TLC, the system was poured into 1L of water, extracted twice with an equal amount of dichloromethane, the organic layer was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the intermediate 11 g of 5-fluoro-1-methyl-1H-indole.
[0090] Add 2,4-di...
Embodiment 2
[0098] Example 2: N-(5-(4-(5-fluoro-1-methyl-1H-indol-3-yl)-5-methylpyrimidin-2-ylamino)-4-methoxy - Synthesis of 2-(methyl(2-(methylamino)ethyl)amino)phenyl)acrylamide (compound 2):
[0099]
[0100] Dissolve compound 1 (2.0 g) obtained in Example 1 in 8 ml of dichloromethane, add 4 ml of trifluoroacetic acid, stir at room temperature for 2 h, TLC detects that the reaction of the raw materials is complete, cool to below 0 degrees Celsius, add 80 ml of saturated sodium bicarbonate and 80 ml of Dichloromethane, stirred for 30 minutes, separated, the aqueous phase was extracted 3 times with an equal amount of dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the target product (compound 2) (1.53g, yield 91 %).
[0101] 1 H-NMR (400MHz, DMSO-d6, δppm): 10.38(s,1H), 9.90(s,1H), 9.63(s,1H), 8.62(s,1H), 8.15(s,2H), 7.59( m,1H),7.30(m,1H),7.16(s,1H),7.00(s,1H),6.18(d,1H,J=16.8Hz),5.78(s,1...
Embodiment 3
[0103] Example 3: N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-5-(4-(5-fluoro-1-methyl-1H-indole- 3- Synthesis of (yl)-5-methylpyrimidin-2-ylamino)-4-methoxy-phenyl)acrylamide (compound 3):
[0104]
[0105] a. N 1 -(2-(Dimethylamino)ethyl)-N 4 -(4-(5-fluoro-1-methyl-1H-indol-3-yl)-5-methylpyrimidin-2-ylamine base)-5-methoxy-N 1 -Synthesis of methylbenzene-1,2,4 triamine:
[0106]
[0107] N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(5-fluoro-1-methyl-1H-indol-3-yl)-5-methylpyrimidine- 2-amine (see Example 1 for the synthesis method) (7g, 1eq), trimethylethylenediamine dihydrochloride (5.7g, 2eq), triethylamine (8.3g, 5eq), trifluoroethanol (100ml) Add it into a sealed tube, raise the temperature to 100 degrees Celsius, and gradually dissolve as the reaction progresses. After about 8 hours, the reaction is completed by TLC and liquid mass monitoring, and concentrated to dryness under reduced pressure to obtain 7.5 g of crude product, which is directly used in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com