Process and system for facilitating chemical identification in detector
A technology for chemical substances and detection parameters, which is used in the calibration of instruments, instruments, measuring devices, etc., and can solve the problem of ions not being completely resolved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
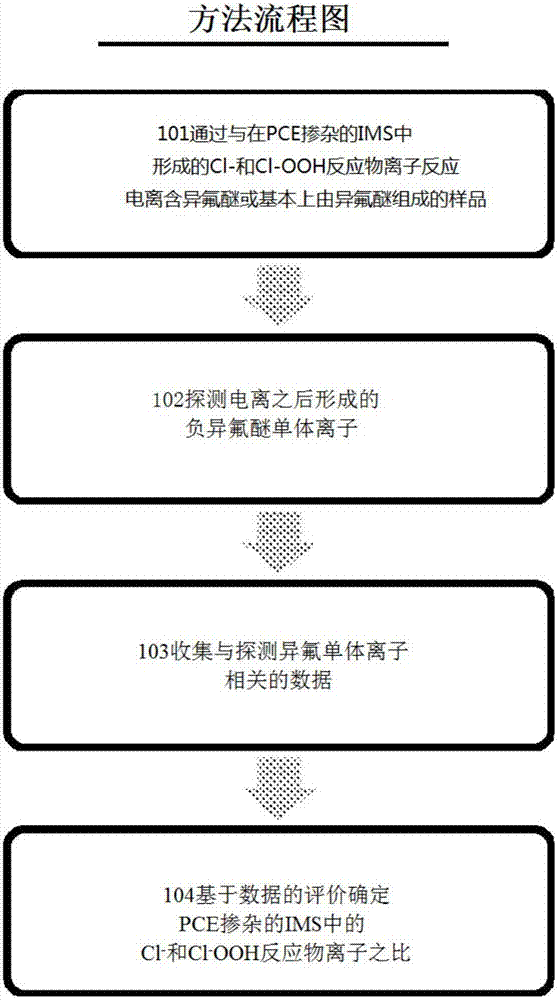
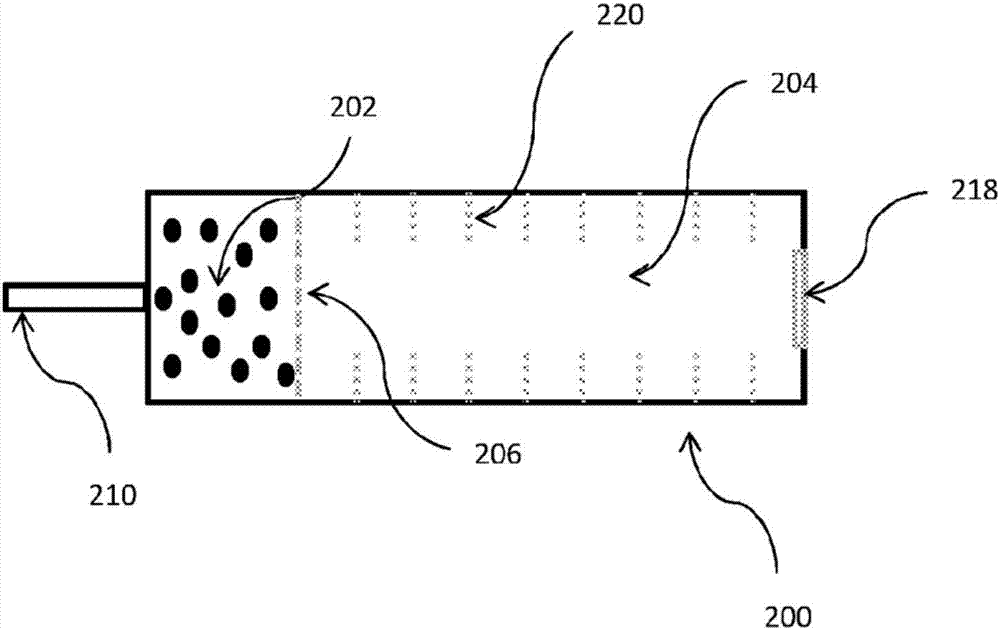
[0027] exist figure 1 In the example shown in , the first part 101 of the method involves the ionization of a sample containing or consisting essentially of isoflurane in a PCE-doped IMS detector to form the PCE-associated reactant ion Cl – and Cl – .OOH. Isoflurane, whose chemical structure is shown below, is known for its use as an anesthetic, is frequently used in veterinary anesthesia, and usually exists as a racemic mixture of (R) and (S) optical isomers .
[0028]
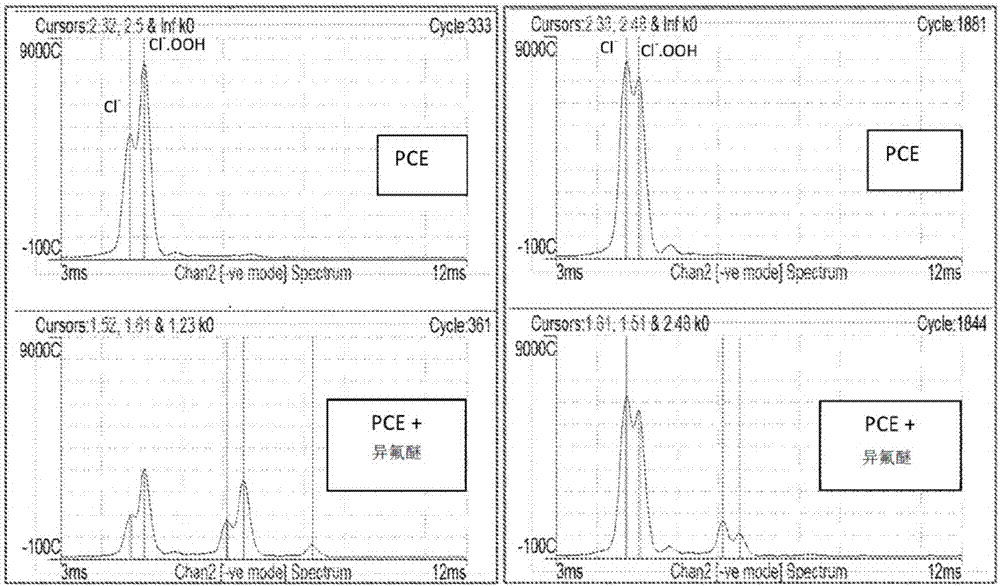
[0029] Isoflurane is particularly advantageous for its PCE-related reactant ion Cl – and Cl – .OOH reacts with both without significant bias towards producing distinct isoflurane monomer ions. Thus, a sample containing or consisting essentially of isoflurane is introduced into a PCE-doped IMS detector, which reacts with the PCE-associated Cl- and Cl-.OOH reactant ions present in the reaction zone to be ionized. After ionization, isoflurane forms a negative monomer ion. The negative isoflurane monom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com