Application of SFN (sulforaphane) to preparation of medicine for treating leukemia
A technology of sulforaphane and leukemia, which is applied in the field of application of sulforaphane in the treatment of leukemia drugs, and can solve the problems of less reports on the role and mechanism of hematopoietic tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
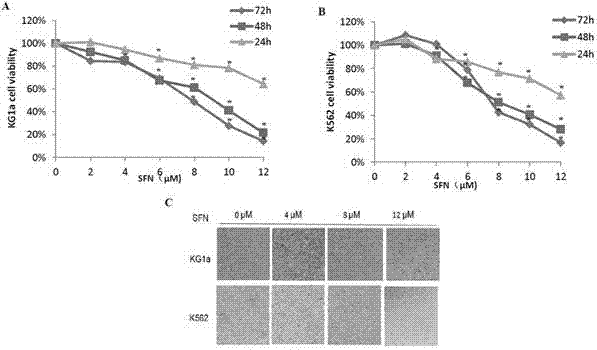
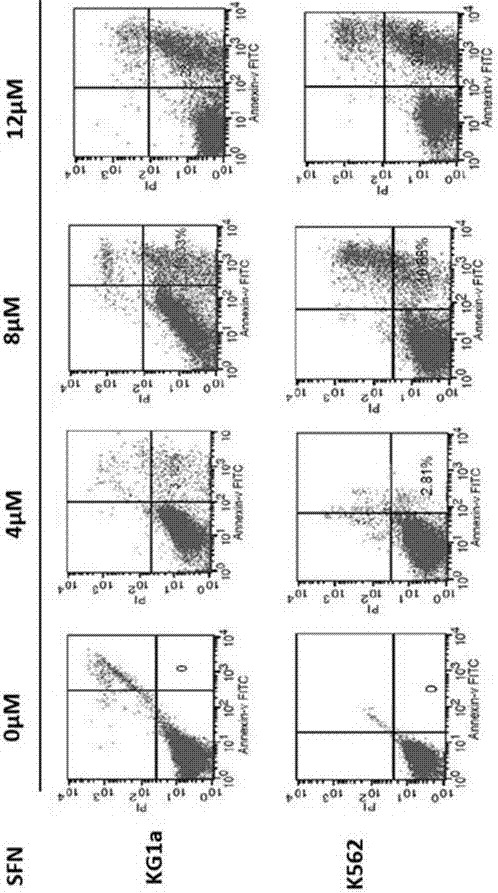
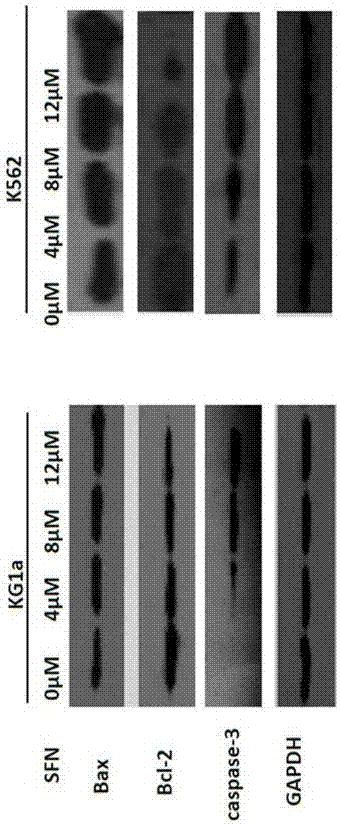
[0017] 1. Experimental materials
[0018] Sulforaphane (batch number 28911701) was purchased from LKT Laboratories, USA, with a purity of 98%. 1640 culture medium and fetal bovine serum are products of Gibco Company in the United States. CCK-8 kit was purchased from Dojindo Company in Japan, Annexin-V / PI apoptosis detection reagent (BD Company, USA, batch number 5121706), RNA extraction kit, reverse transcription kit, PCR reagent 2×Taq PCRMaster Mix, DNA Marker All were purchased from Shanghai Tiangen, agarose (Biowest, Spain), primers were synthesized by Shanghai Bioengineering Company, rabbit anti-human Bax mAb, rabbit anti-human Bcl-2 mAb, rabbit anti-human Caspase-3 mAb were produced by American Abcam Company, anti-human Human GAPDH pAb and HRP-goat anti-rabbit pAb were provided by Century Kangwei Company. The flow cytometer (FACSCaibur type) is produced by BD Company in the United States, the MK3 microplate reader and ND2000 ultra-micro spectrophotometer are produced by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com