18F-labeled polypeptide tumor apoptosis detection reagent, and preparation method and application thereof
A 18F and labeling technology, which is applied in the fields of radiopharmaceuticals and nuclear medicine, can solve the problems of long labeling time, harsh reaction conditions, cumbersome steps, etc., and achieve the effect of easy separation, mild reaction conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
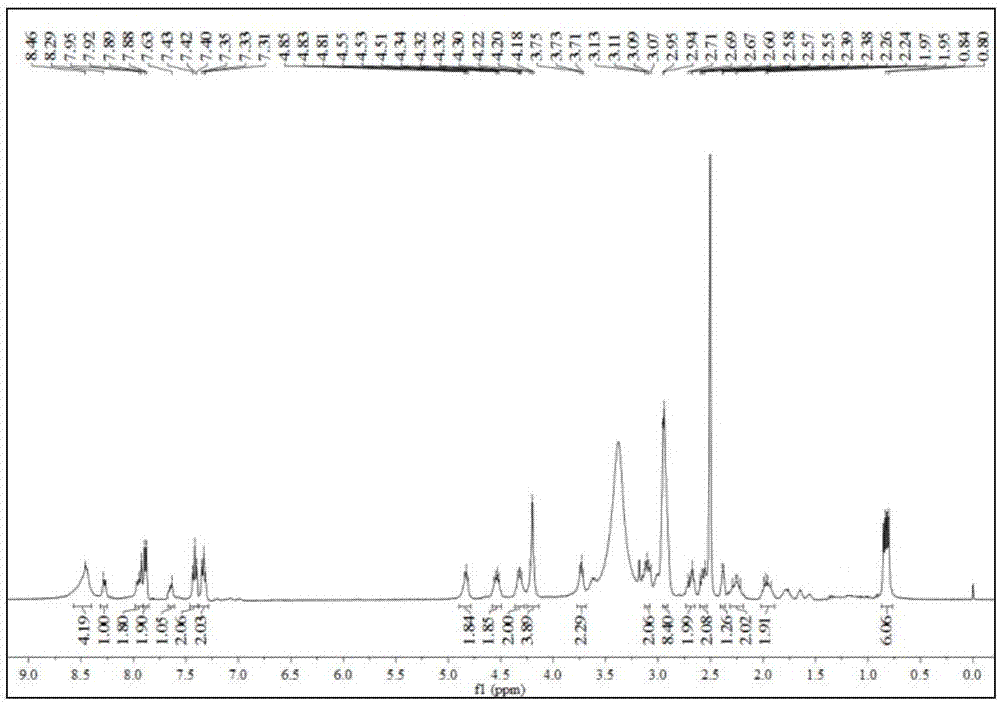
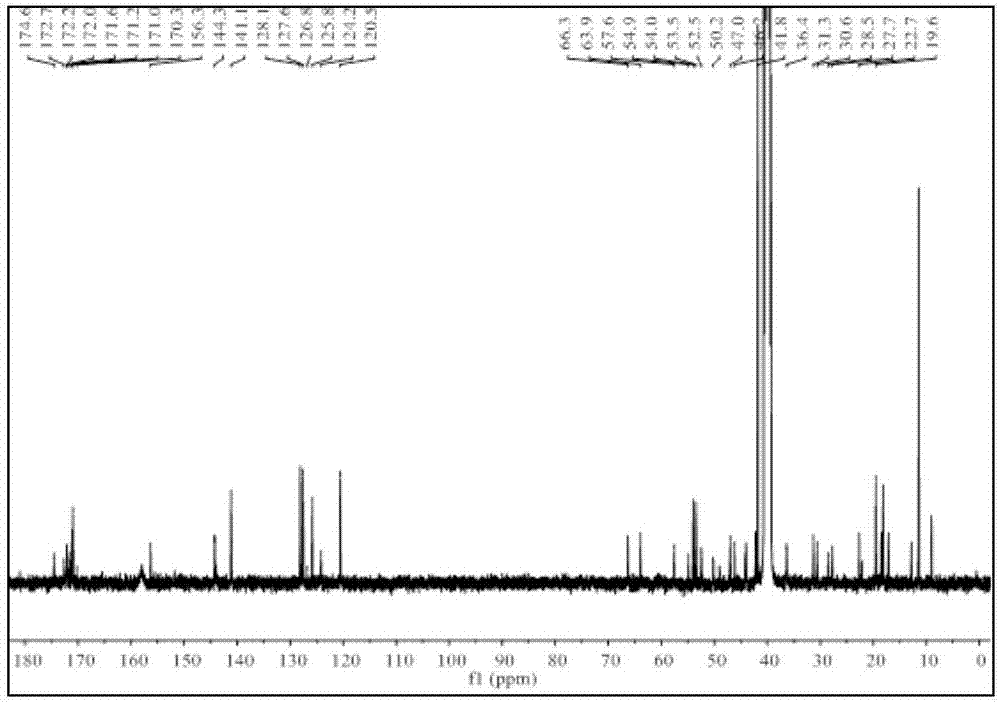
[0051] Synthesis of Example 1 Labeled Precursor
[0052] The synthetic route of labeled precursor 1 described in this example is as follows:
[0053]
[0054] The synthesis of the labeled precursor 1 described in this example comprises the following steps:
[0055] (1) Connecting amino acids: Weigh 2-chlorotrityl chloride resin (544mg, Loading value: 1.106mmol / g) into a three-way sand core funnel, then add 10mL of dichloromethane (DCM) solvent, shake for 10min , drain the solvent;
[0056] Fmoc-L-aspartic acid beta-tert-butyl ester (272mg, 0.662mmol) was weighed and dissolved in 3mL DMF solvent, then DIPEA (200μL, 1.212mmol) was added, ultrasonically dissolved and added to a sand core funnel to react with the resin, and Shake at room temperature for 2 h; then drain the solvent, and add 7 mL of solution R (chromatographically pure DMF: CH 3 OH:DIPEA=17:2:1) Wash and shake in the three-way sand core funnel for 10min, drain the solvent, then wash with 7mL DMF solvent, then ...
Embodiment 2
[0077] Embodiment 2 polypeptide compound 18 F-AmBF 3 - Synthesis of GDEVD
[0078] Electron bombardment of oxygen-enriched water H 2 18 O, get 18 F ion, adsorbed by anion exchange column (QMA) 18 For F ions, use 300 μL of pyridazine (pH=2.5) buffer to 18 F ions were eluted from the QMA column, transferred to a 1 mL labeled reaction tube, added 10-20 μL (25 mmol / L, dissolved in DMF) of the labeled precursor compound 1 prepared in Example 1, and heated at 80 °C 30min reaction to get the peptide compound 18 F-AmBF 3 -GDEVD, denoted as probe 18 F-1.
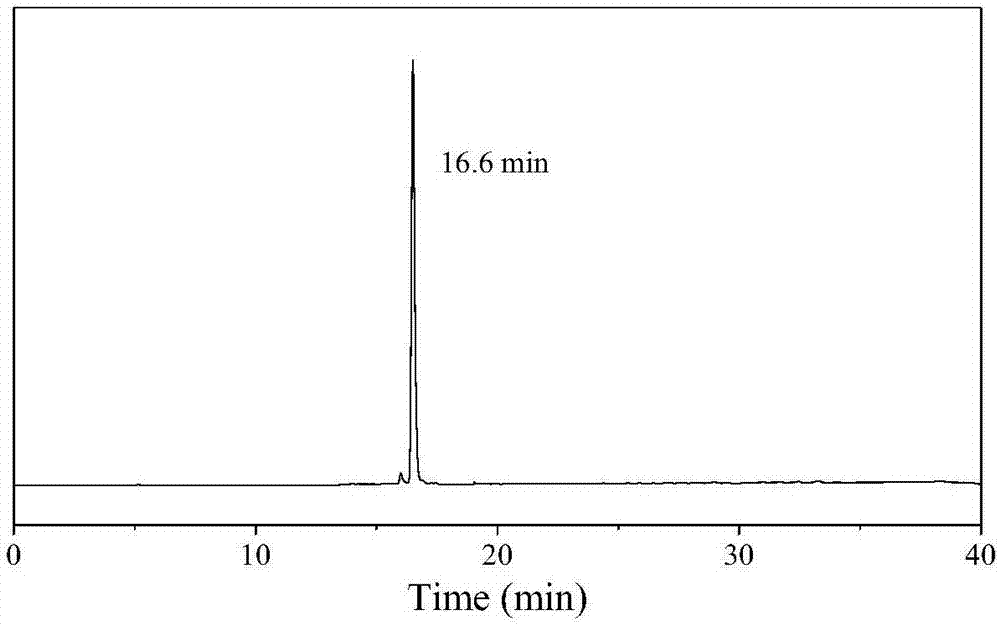
[0079] Dilute a small amount of radioactive solution, and detect the labeling of the precursor by radioactive high-performance liquid phase. Add 20 mL of deionized water to the reaction solution for dilution, pass through a C18 column (SepPak plus C-18), wash with deionized water (10 mL) three times, and finally use 0.5 mL of ethanol to rinse the labeled product into a vial, add 4.5 Dilute with mL deionized water (ethanol ...
experiment example
[0083] 1. Determination of stability in PBS and serum
[0084] In order to simulate the in vivo environment, the 18 Stability of F-1 in PBS (pH=7.4) and serum. Take 4 parts of 400 μL PBS (pH=7.4) buffer and 100 μL labeled product 18 The F-1 solutions were mixed separately and heated at 37°C for 0.5, 1, 2, 3 and 4 h, respectively. A small amount of solution was taken for dilution, and the stability of the labeled product was detected by radioactive high-performance liquid phase.
[0085] Take 4 aliquots of 400 μL FBS and 100 μL labeled product 18 The F-1 solutions were mixed separately and incubated at 37°C for 0.5, 1, 2, 3 and 4 hours respectively. After the time point, 500 μL of acetonitrile was added, centrifuged at 12,000 g / min for 5 min with a high-speed centrifuge, and the supernatant was taken, and the stability of the labeled product was detected by radioactive high-performance liquid chromatography.
[0086] Detection of different incubation time points by radioac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com