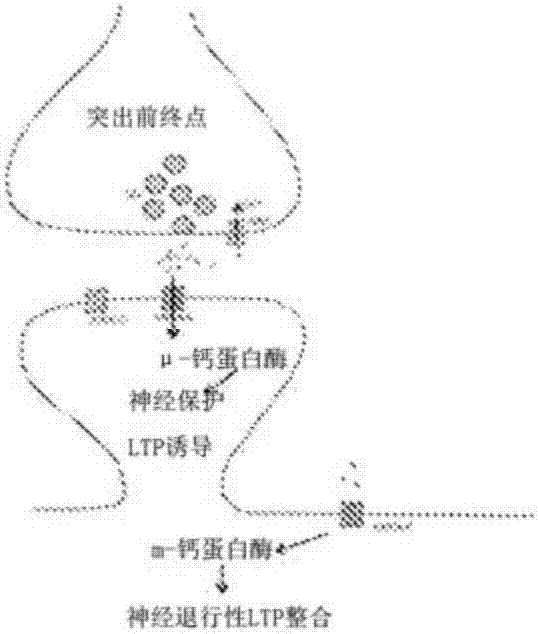
Isoform-specific calpain inhibitors, methods of identification, and uses thereof
An excipient, pharmaceutical technology, applied in the field of isotype-specific calpain inhibitors and their identification and use, can solve the problem of not creating inhibitors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Example 1 Small Molecules / Modified Polypeptides with High Selectivity for Calpain-2
[0179]
[0180] Formula 1, in which chiral center 1 is the L-form and in which chiral center 2 is a racemic mixture of D- and L-, was introduced in vitro at different concentrations (from 1 nM to 10 μM) mixed, including succinyl-leucine-tyrosine-AMC and calpain-1 or calpain-2 (Sasaki et al., 1984), and the kinetics of fluorescence loss for each calpain was determined (Powers et al., 2000 ). The Ki values of the compounds obtained in the literature are 2.3 μM for calpain-1 and 0.22 μM for calpain-2 (Li et al., 1996). However, the present inventors re-determined the Ki of calpain-1 as 1.29 μM±0.7 μM and the Ki of calpain-2 as 0.025 μM±0.02 μM. Accordingly, the selectivity assessment described herein differs from previous teachings. This compound is a highly selective inhibitor of calpain-2 as its Ki for calpain-2 is more than 50 times lower than the Ki for calpain-1.
Embodiment 2
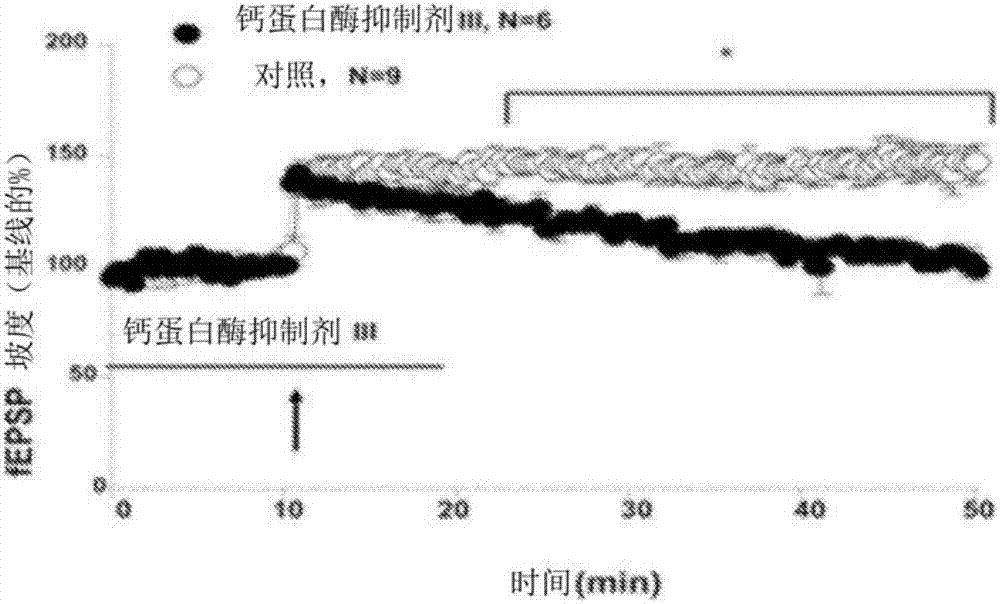
[0181] Example 2: Universal calpain inhibitors block LTP when administered prior to LTP induction. Excitatory postsynaptic potentials (EPSP, figure 2 ) field records. 10 μM calpain inhibitor III (Z-Val-Phe-CHO, with a Ki of about 8 nM for calpain-1 and calpain-2), which inhibits both calpain-1 and calpain-2, was stimulated by theta rhythm ( TBS, 10 rhythms of 4 pulses at 100 Hz with a rhythm interval of 200 ms) was added before, which can be used to elicit LTP (Capocchi et al., 1992). When compared to control (compare open and filled circles), pre-incubation of a non-selective calpain inhibitor (calmodulinase inhibitor III) did not prevent short-term potentiation (increased fEPSPs after TBS), but prevented LTP formation .
Embodiment 3
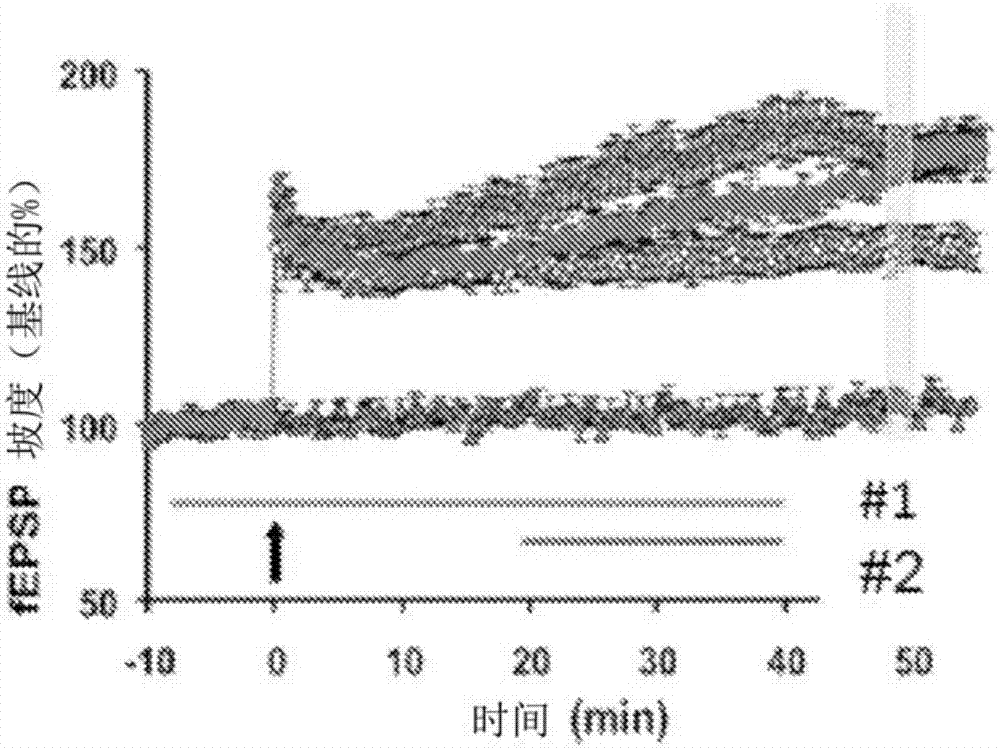
[0182] Example 3: Calpain 2-selective inhibitors enhance LTP. Acute hippocampal slices were prepared and soaked in ACSF. After being able to induce LTP (see Figure 3A 200 nM of the calpain-2 selective inhibitor of formula 1, which is 50-100 times more inhibiting of calpain-2 than calpain-1, was administered prior to theta-rhythmic stimulation of time-dependent administration of line #1. In contrast to the unexpected administration of non-selective calpain inhibitors such as calpain inhibitor III, pre-incubation with calpain-2 selective inhibitor pre-incubation did not inhibit LTP ( Figure 3A ); instead, LTP is enhanced. Incubation of hippocampal slices with the same highly selective calpain-2 inhibitor after theta-rhythmic stimulation (TBS) also resulted in an increase in LTP during the TTP immobilization phase when administered from 10 min post-TBS to 1 hr post-TBS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com