Method for modifying ligands on surfaces of cell vesicles
A technology of surface modification and microvesicles, applied in cell membrane modification, biochemical equipment and methods, microorganisms, etc., can solve problems such as the influence of membrane structure and physicochemical properties, the modification of non-cellular microvesicles, and the limitation of research and application progress. , to reduce the uptake by phagocytes, save the amount of ligands, and maintain the effect of physiological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
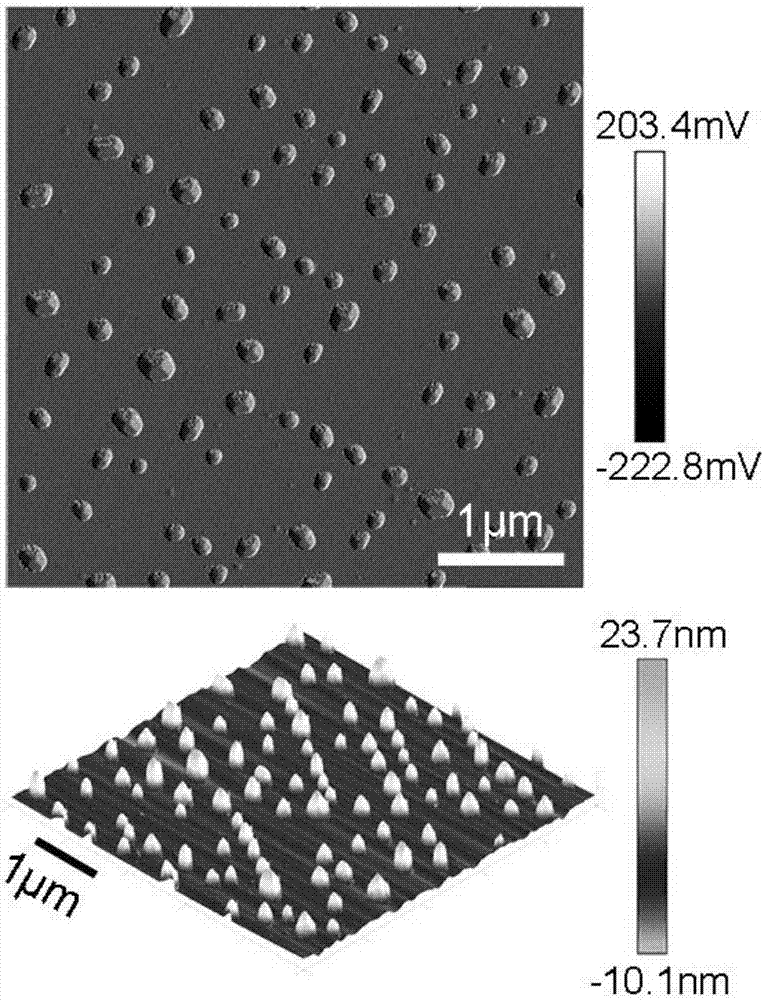
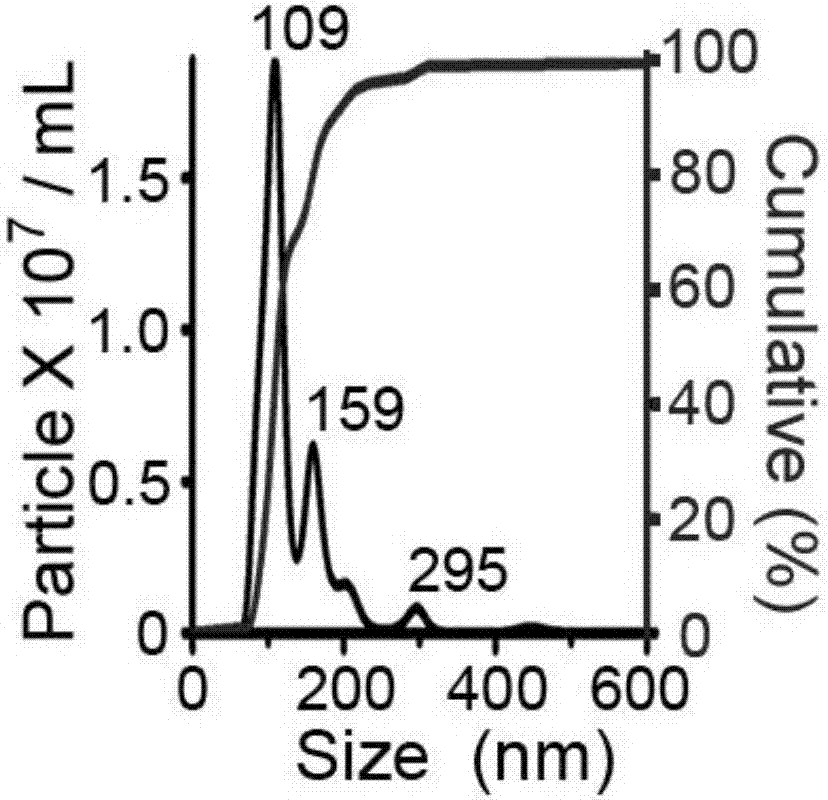
Image
Examples
Embodiment 1
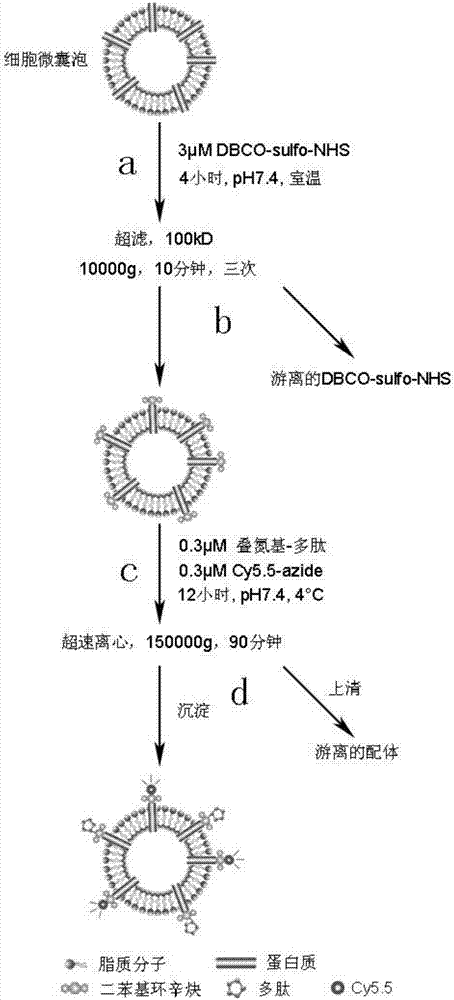
[0035] Example 1, RGD polypeptide modified on the surface of exosomes.
[0036] Such as figure 1 shown.
[0037] Step (a), the 1×10 12 Exosomes were resuspended in 1 mL pH7.4 PBS and added to a 1.5 mL centrifuge tube. Then 3 μM DBCO-sulfo-NHS was added. The particle number / mole ratio of exosomes to DBCO-sulfo-NHS is 10 12 : 3 nmol, the centrifuge tube was fixed on a rotary mixer, and reacted at room temperature for 4 hours.
[0038] In step (b), add the solution obtained in step (a) to the upper layer of two ultrafiltration tubes (pore size 100kD, capacity 0.5mL), and centrifuge at 10000g for 10 minutes. The lower layer filtrate was discarded, and the upper layer was supplemented with PBS at pH 7.4 to a volume of 0.5 mL. The ultrafiltration was repeated twice according to the same procedure to obtain 1 mL of DBCO-modified exosomes.
[0039] In step (c), the solution obtained in step (b) was placed in a 1.5 mL centrifuge tube. Add 0.3 μM azido-modified RGD polypeptide (th...
Embodiment 2
[0042] Example 2, EGFR antibody modified on the surface of microvesicles.
[0043] Step (a), the 1×10 13 The microvesicles were resuspended in 10 mL of PBS, pH 7.4, and added to a 15 mL centrifuge tube. Then add 8 μM DBCO-NHS. The particle number / mole ratio of microvesicles to DBCO-NHS is 10 12 : 8nmol, the centrifuge tube was fixed on the rotary mixer, and reacted at room temperature for 6 hours.
[0044] In step (b), add the solution obtained in step (a) to the upper layer of two ultrafiltration tubes (pore size 300kD, capacity 5mL), and centrifuge at 12000g for 10 minutes. The lower layer filtrate was discarded, and the upper layer was supplemented with PBS at pH 7.4 to a volume of 5 mL. The ultrafiltration was repeated twice according to the same procedure to obtain 10 mL of DBCO-modified microvesicles.
[0045] In step (c), the solution obtained in step (b) is placed in a 15mL centrifuge tube. Add 2 μM azide-modified EGFR antibody (the azide-modified antibody molecu...
Embodiment 3
[0048] Example 3, siRNA modified on the surface of extracellular vesicles.
[0049] Step (a), the 1×10 14 Extracellular vesicles were resuspended in 1 mL pH7.4 PBS and added to a 1 mL centrifuge tube. Then 1 mM DBCO-PEG4-NHS was added. The particle / mole ratio of extracellular vesicles to DBCO-PEG4-NHS is 10 12 : 10 nmol, the centrifuge tube was fixed on a rotary mixer, and reacted at room temperature for 12 hours.
[0050] In step (b), add the solution obtained in step (a) to the upper layer of two ultrafiltration tubes (pore size 100 kD, capacity 0.5 mL), and centrifuge at 12000 g for 8 minutes. The lower layer filtrate was discarded, and the upper layer was supplemented with PBS at pH 7.4 to a volume of 0.5 mL. Repeat the ultrafiltration three times according to the same procedure to obtain 1 mL of DBCO-modified extracellular vesicles.
[0051] In step (c), the solution obtained in step (b) was placed in a 1 mL centrifuge tube. Add 0.5 mM azido-modified siRNA (the azid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com