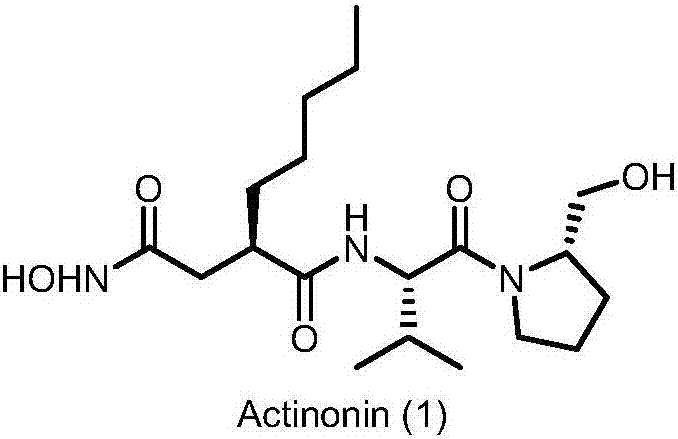
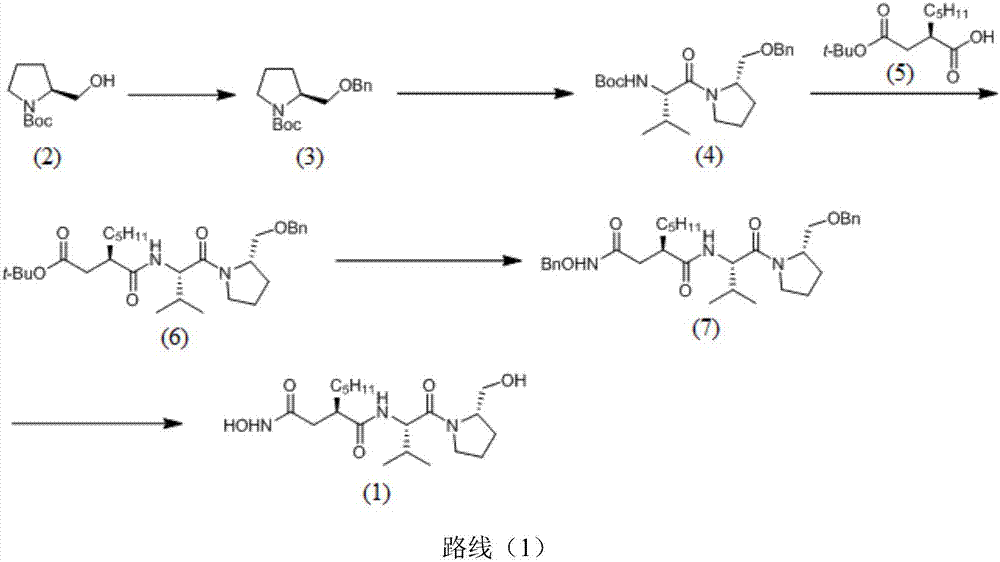
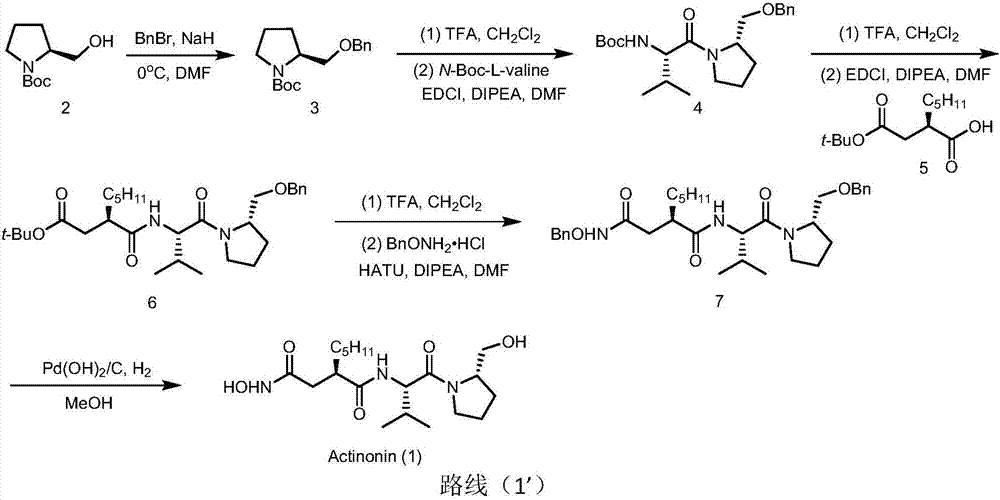
Preparation method of actinonin
A technology of actinamide and amide condensation, applied in the field of actinamide preparation, can solve problems such as inconvenient operation, compound chiral center racemization, difficult to remove by-products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Synthesis of Compound 3
[0082]
[0083] N-Boc-L-prolinol (6.03g, 30mmol) was weighed and dissolved in dry DMF (60mL), under nitrogen protection, and cooled in an ice-water bath. NaH (1.5 g, 37.5 mmol) was added in one portion and stirred for 30 min. Then, BnBr (5.36g, 31.5mmol) was weighed and dissolved in dry DMF (17.5mL), and added dropwise to the above reaction solution within 15min at 0°C. After the addition, the system was naturally raised to room temperature and stirred overnight. Add water to quench the reaction, extract with ethyl acetate (2×100mL), wash the organic phase with water (80mL) and saturated brine (60mL) respectively, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain a light yellow oily crude product , the crude product was separated and purified by column chromatography to obtain compound 3 (7.94 g, 91%) as a colorless oil. 1 H NMR (400MHz, CDCl 3 )δ7.39–7.17(m,5H),4.52(s,2H),3.96(d,J=47.6...
Embodiment 2
[0097] Synthesis of Compound 3
[0098]
[0099] N-Boc-L-prolinol (3.02g, 15mmol) was weighed and dissolved in dry DMF (30mL), under nitrogen protection, and cooled in an ice-water bath. NaH (0.9 g, 22.5 mmol) was added in one portion and stirred for 30 min. Then, BnBr (2.81g, 16.5mmol) was weighed and dissolved in dry DMF (7.5mL), and added dropwise to the above reaction solution at 0°C within 15min. After the addition, the system was naturally raised to room temperature and stirred overnight. Add water to quench the reaction, extract with ethyl acetate (2×50mL), wash the organic phase with water (30mL) and saturated brine (30mL) respectively, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain a light yellow oily crude product , the crude product was separated and purified by column chromatography to obtain compound 3 (3.80 g, 87%) as a colorless oil. 1 H NMR (400MHz, CDCl 3 )δ7.39–7.17(m,5H),4.52(s,2H),3.96(d,J=47.6...
Embodiment 3
[0113] Synthesis of Compound 3
[0114]
[0115] N-Boc-L-prolinol (4.03g, 20mmol) was weighed and dissolved in dry DMF (40mL), under nitrogen protection, and cooled in an ice-water bath. NaH (1.08 g, 27 mmol) was added in one portion and stirred for 30 min. Then, BnBr (3.64g, 21.4mmol) was weighed and dissolved in dry DMF (7.5mL), and added dropwise to the above reaction solution within 15min at 0°C. After the addition, the system was naturally raised to room temperature and stirred overnight. Add water to quench the reaction, extract with ethyl acetate (2×50mL), wash the organic phase with water (30mL) and saturated brine (30mL) respectively, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain a light yellow oily crude product , the crude product was separated and purified by column chromatography to obtain compound 3 (4.54 g, 78%) as a colorless oil. 1 H NMR (400MHz, CDCl 3 )δ7.39–7.17(m,5H),4.52(s,2H),3.96(d,J=47.6H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com