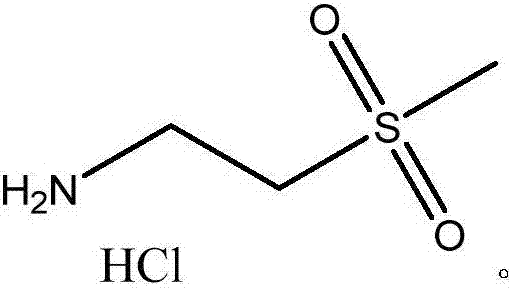
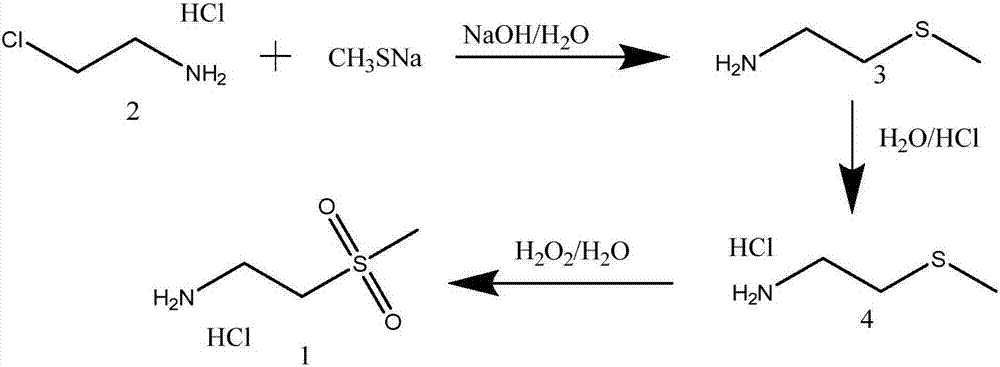
Preparation method of lapatinib intermediate 2-(methanesulfonyl)ethylamine hydrochloride
A technology of ethylamine hydrochloride and chloroethylamine hydrochloride is applied in the field of preparation of lapatinib intermediate 2-ethylamine hydrochloride, which can solve the problems of huge pressure on environmental protection and complicated steps, and achieve less three wastes , The effect of good product quality, simple and easy price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] 1) Synthesis of 2-(methylthio)ethylamine
[0031] In a 2000ml three-neck flask equipped with mechanical stirring, add 2-chloroethylamine hydrochloride (116g, 1mol) and 240ml of water, stir until dissolved, cool down to 0-5°C in an ice-salt bath, and keep the temperature at 0-5°C. 30% sodium hydroxide solution (150 g, 1.12 mol) was added dropwise. After the addition was complete, the stirring reaction was continued for 30 min, and the pH value of the reaction solution was greater than 12. Keep the reaction temperature at 0-5°C, add 20% sodium methyl mercaptide solution (380g, 1.1mol) dropwise for 0.5-1h, keep the reaction at room temperature for 10-12 hours, when the reaction is complete, extract with 300ml×3 dichloromethane, The organic layer was washed twice with 1000 ml of water, and dried over anhydrous magnesium sulfate. After the solvent was recovered under normal pressure, the distillation was continued to collect fractions at 146-148° C. to obtain 2-(methylthio)...
example 2
[0035] Step 2) of Example 1 adopts acetone, n-hexane, cyclohexane, a 1:1 mixed solvent of acetone and n-hexane, and isopropyl ether as the refining solvent instead of ethanol for refining. The product purity and yield are listed as follows:
[0036] Table 1 product purity and yield
[0037] solvent Product purity (GC method) product yield acetone 99.7% 83.3% n-Hexane 99.8% 85.5% Cyclohexane 99.6% 87.3% Acetone / n-hexane=1:1 99.7% 88.6% isopropyl ether 99.8% 82.0%
example 3
[0039] 1) Synthesis of 2-(methylthio)ethylamine
[0040] In a 2000ml three-neck flask equipped with mechanical stirring, add 2-chloroethylamine hydrochloride (116g, 1mol) and 250ml of water, stir until dissolved, cool down to 0-5°C in an ice-salt bath, and keep the temperature at 0-5°C. 30% sodium hydroxide solution (160 g, 1.20 mol) was added dropwise. After the addition was complete, the stirring reaction was continued for 30 min, and the pH value of the reaction solution was greater than 12. Keeping the reaction temperature at 0-5°C, add 20% sodium methyl mercaptide solution (380g, 1.1mol) dropwise, after 40min, keep the reaction at room temperature for 10 hours. The water was washed twice with water, and dried with anhydrous magnesium sulfate. After recovering the solvent under normal pressure, the distillation was continued to collect fractions at 146-148° C. to obtain 2-(methylthio)ethylamine as 85.8 g of a colorless liquid with an amine smell, and the yield was 94.2%. ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap