Methods and compositions for stabilizing trans-splicing intein modified proteases
A trans-splicing and intein technology, applied in detergent compositions, fusion with post-translational modification motifs, chemical instruments and methods, etc., can solve the problems of increasing chemical load and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
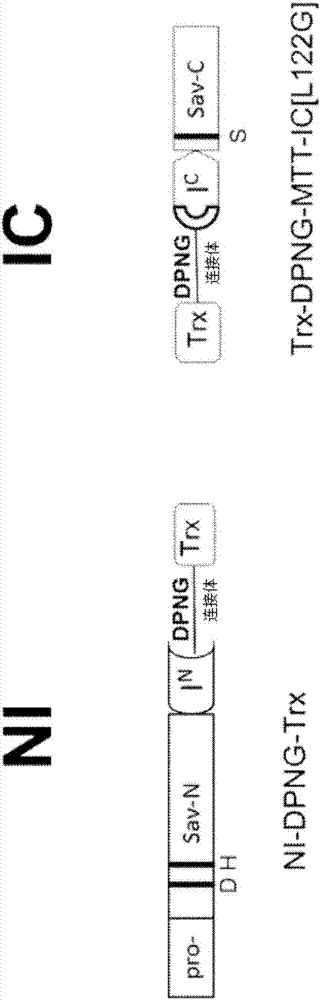
[0059]One embodiment includes a composition comprising the precursors (NI and IC) of any of the intein-modified proteases herein. The first precursor NI may contain, consist essentially of, or consist of at least 70, 72, 75, 80, 85, 90, 91, 92, 93, 94, 95, 96, 97 of the reference sequence of SEQ ID NO: 1 , 98, 99 or 100% identity amino acid sequence composition. The first precursor may be capable of trans-splicing with the second precursor to form the target protease. The second precursor IC may contain, consist essentially of, or consist of at least 70, 72, 75, 80, 85, 90, 91, 92, 93, 94, 95, 96, 97 of the reference sequence of SEQ ID NO: 2 , 98, 99 or 100% identity amino acid sequence composition. The second precursor may be capable of trans-splicing with the first precursor to form the target protease. The concentration of the first precursor or the second precursor may be in the range of 0.01% (v:v) to 10% (v:v). Concentration can be 0.02%(v:v), 0.03%(v:v), 0.04%(v:v),...
Embodiment 1
[0169] Example 1. Trans-splicing Intein Technology for Modulating Protease Activity
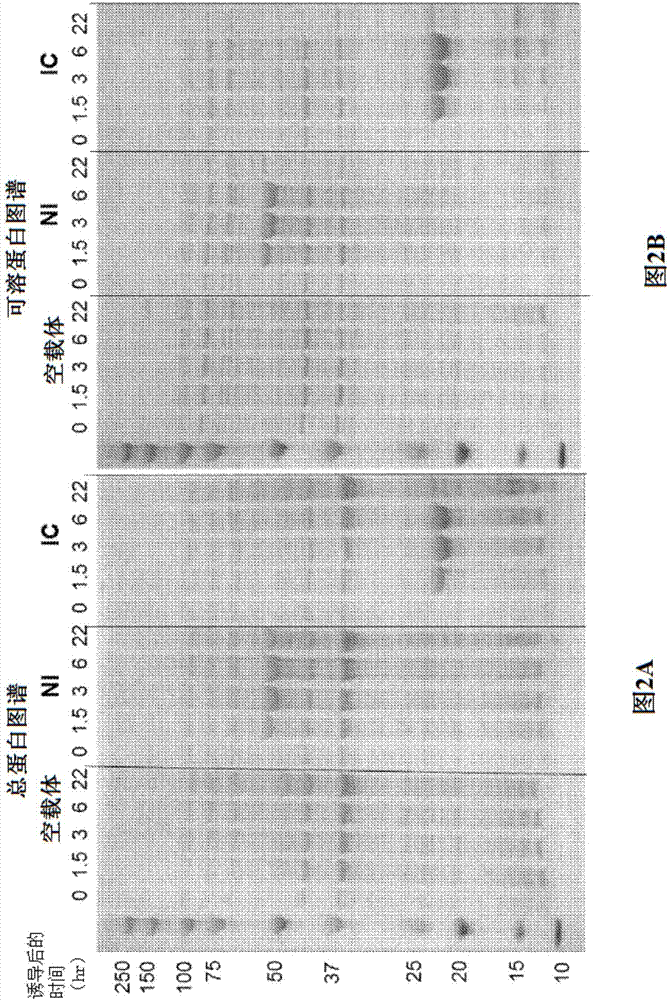
[0170] Trans-splicing intein technology can be used to modulate protease activity. In this approach, the protease is split between the catalytic residues into two inactive fragments, each of which is expressed as a fusion to a trans-spliced intein. Mixing in an aqueous environment triggers trans-intein-mediated conjugation of the inactive fragment, splicing and seamless ligation of the inactive portion to form a fully functional active protease.
[0171] The construction, expression and detergent dilution-inducing properties of the trans-spliced intein-regulated liquid laundry protease Savinase have been described in PCT patent application PCT / US2013 / 063304, which is incorporated herein by reference as if fully set forth. Described herein are methods to improve formulation stability and detergent dilution-inducible protease activity to levels relevant for commercial liquid laundry applic...
Embodiment 2
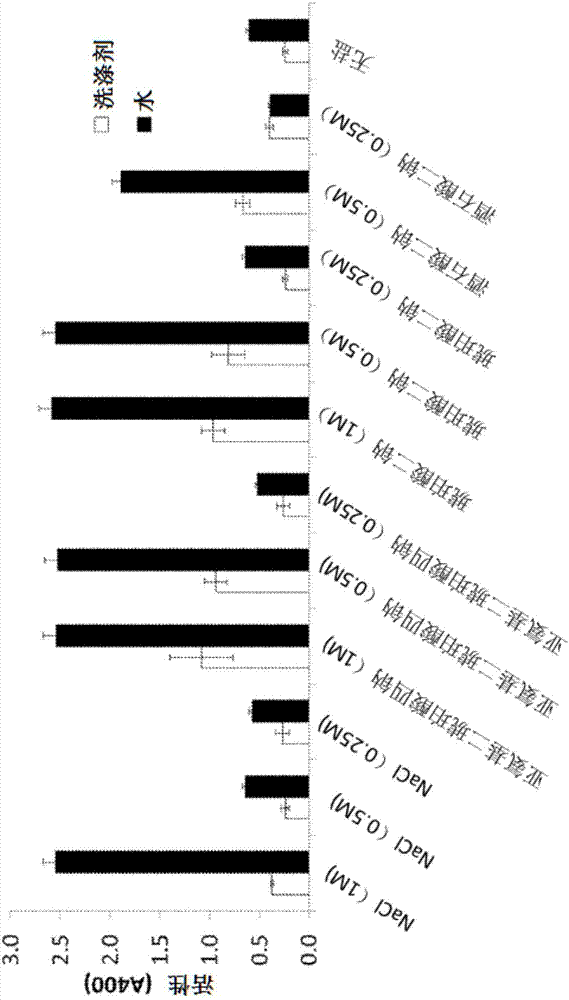
[0172] Example 2. Molecules optimized for solubility
[0173] Detergent refers to liquid laundry detergent. Detergents-1 and -4 are standard liquid detergent formulations without enzymes, optical brighteners and colorants. Detergent-1 can contain Deionized Water, Neodol 25_7, LAS Acid, Prifac 5908, Dequest 2010, SLES 3EO, Sodium Sulfite, Sokalan HP20, MPG, MEA, TEA, Glycerin, Tinopal 5BMGX and other common liquid laundry detergent ingredients . Detergent-1 had a pH of ~8.3±0.2 and a conductivity of ~3890 μSimens. Detergent-4 had a pH of ~7.3±0.2 and a conductivity of ~22400 μSimens.
[0174] Detergents-2 and -3 were commercial brand detergents with formulated protease free fluorescers and colorants. Detergent-2 had a pH of ~8.8±0.2 and a conductivity of ~3900 μSimens. Detergent-3 had a pH of ~8.3±0.2 and a conductivity of ~7090 μSimens. Proteases were inactivated by heat treatment at 80°C for 90 min. After cooling to room temperature, verify the absence of proteases as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com