Method for preparing metal organic framework material UiO-66 in ethanol phase
A metal-organic framework and organic framework technology are applied in the preparation of metal-organic framework material UiO-66, and in the field of preparation of metal-organic framework material UiO-66 in ethanol phase under normal temperature conditions, which can solve the limitation of amplification reaction, increase production cost, Difficulty and other problems, to achieve the effect of reducing synthesis cost, reducing usage and overcoming low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
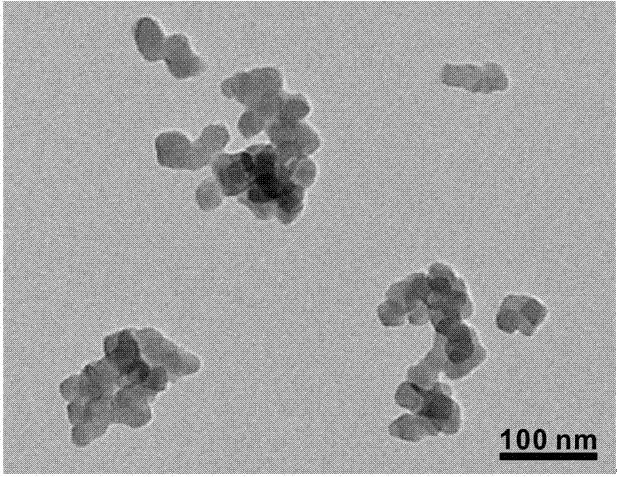
Image
Examples
Embodiment 1
[0035] Weigh 0.291 gZrCl respectively 4, 0.208 g of terephthalic acid in a mortar, grind vigorously for 10 minutes to make it evenly mixed, then transfer the mixture into a 10 mL ball milling jar, the volume ratio of large, medium and small ball milling beads is 1:6:3, the ball milling beads Accounting for 1 / 2 of the volume of the ball milling jar, the ball milling beads and the ball milling jar are made of alumina ceramics, milled at 420rpm for 2 h, take out the ball-milled mixture, add 5 mL of ethanol, and ultrasonicate for 15 minutes, at 600 The reaction was carried out at a stirring speed of rpm for 48 h.
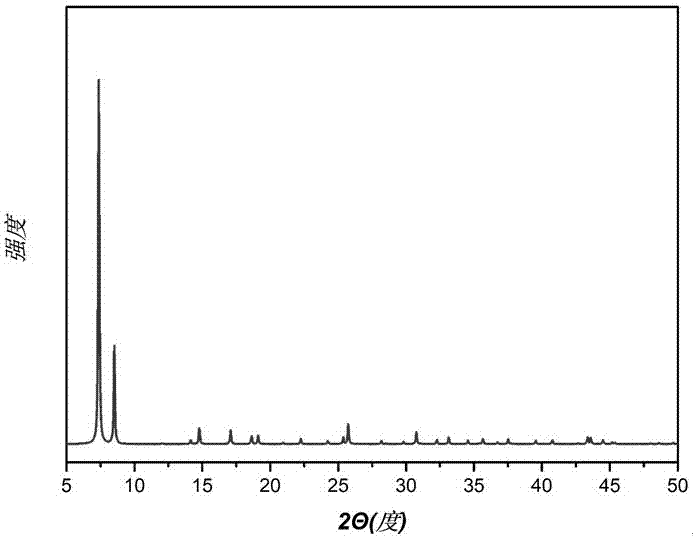
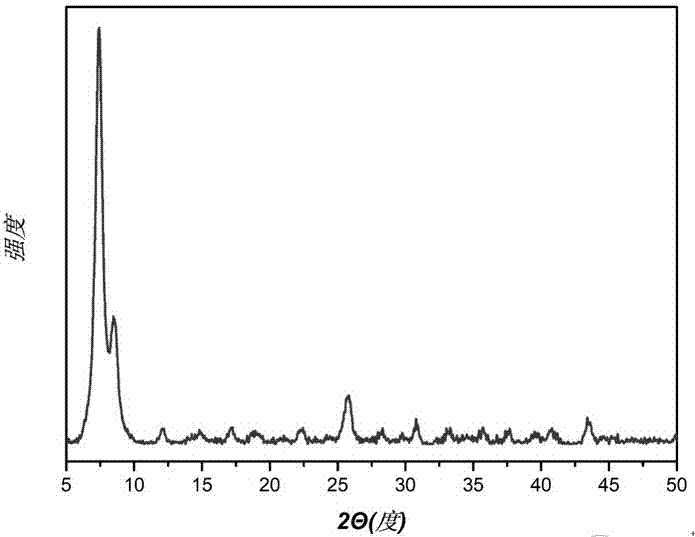
[0036] The crude product after the reaction was obtained by centrifugation, washed with ethanol several times, and the washed crude product was vacuum-dried to obtain 0.157 g of the product with a yield of 51%. Through XRD analysis, its characteristic peak (see figure 2 ) and theoretically simulated characteristic peaks of UiO-66(Zr) (see figure 1 ) are consistent, ...
Embodiment 2
[0039] Weigh 0.402 gZrOCl respectively 2 •8H 2 O. 0.208 g of terephthalic acid was placed in a mortar, ground vigorously for 10 min to make it evenly mixed, and then the mixture was transferred into a 10 mL ball milling jar, the volume ratio of large, medium and small ball milling beads was 1:6:3, and ball milling Beads accounted for 1 / 2 of the volume of the ball milling jar, and the materials of both the ball milling beads and the ball milling jar were alumina ceramics. They were ball-milled for 2 h at a speed of 420 rpm, and the mixture that had been ball-milled was taken out, and 5 mL of ethanol was added. After ultrasonication for 15 minutes, the The reaction was carried out for 48 h at a stirring speed of 600 rpm.
[0040] The reacted crude product was obtained by centrifugation, washed with ethanol several times, and the washed crude product was vacuum-dried to obtain 0.160 g of the product with a yield of 52%. Through XRD analysis, its characteristic peak (see Figur...
Embodiment 3
[0042] Weigh 0.444gZr(SO 4 ) 2 • 4H 2 O. 0.208 g of terephthalic acid was placed in a mortar, ground vigorously for 10 min to make it evenly mixed, and then the mixture was transferred into a 10 mL ball milling jar, the volume ratio of large, medium and small ball milling beads was 1:6:3, and ball milling Beads accounted for 1 / 2 of the volume of the ball milling jar, and the materials of both the ball milling beads and the ball milling jar were alumina ceramics. They were ball milled for 2 h at a speed of 420 rpm, and the mixture that had been ball milled was taken out, and 5 mL of ethanol was added. After ultrasonication for 15 minutes, the The reaction was carried out for 48 h at a stirring speed of 600 rpm.
[0043] The reacted crude product was obtained by centrifugation, washed with ethanol several times, and the washed crude product was vacuum-dried to obtain 0.165 g of the product with a yield of 52%. Through XRD analysis, its characteristic peak (see Figure 5 ) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com