Application of deferoxamine to preparation of medicine for treating nonalcoholic fatty liver
A technology for preparing drugs and deferoxamine, which can be used in drug combinations, active ingredients of amides, digestive system, etc., and can solve problems such as difficult adherence of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Desferoxamine mesylate used in Example 1 and Example 2 is Desfer (deferoxamine mesylate for injection), which is white to off-white loose block or powder, Novartis Pharma Stein AG (Swiss Novartis Pharmaceutical Co., Ltd.), import drug registration certificate number H20140678. The deferoxamine mesylate used in Example 3 was produced by Sigma-Aldrich (Shanghai) Trading Co., Ltd., product number D9533.
[0046] The structural formula of deferoxamine mesylate is shown in formula (I).
[0047]
[0048] Desferoxamine mesylate solution: dissolve deferoxamine mesylate in sterilized 0.9% physiological saline solution, so that the concentration of deferoxamine mesylate is 0.00625 g / ml.
[0049] HepG2 cells (human liver cancer cells): American Type Culture Collection.
[0050] Embodiment 1, animal test
[0051] 1. Group processing
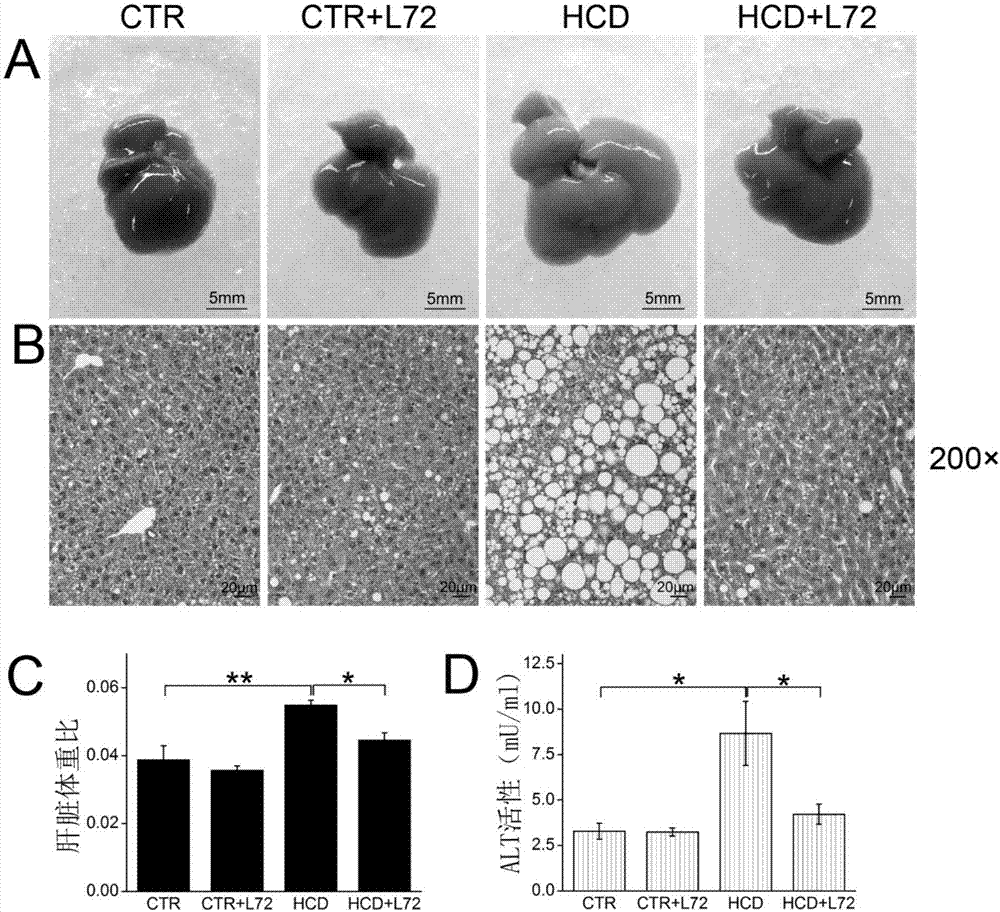
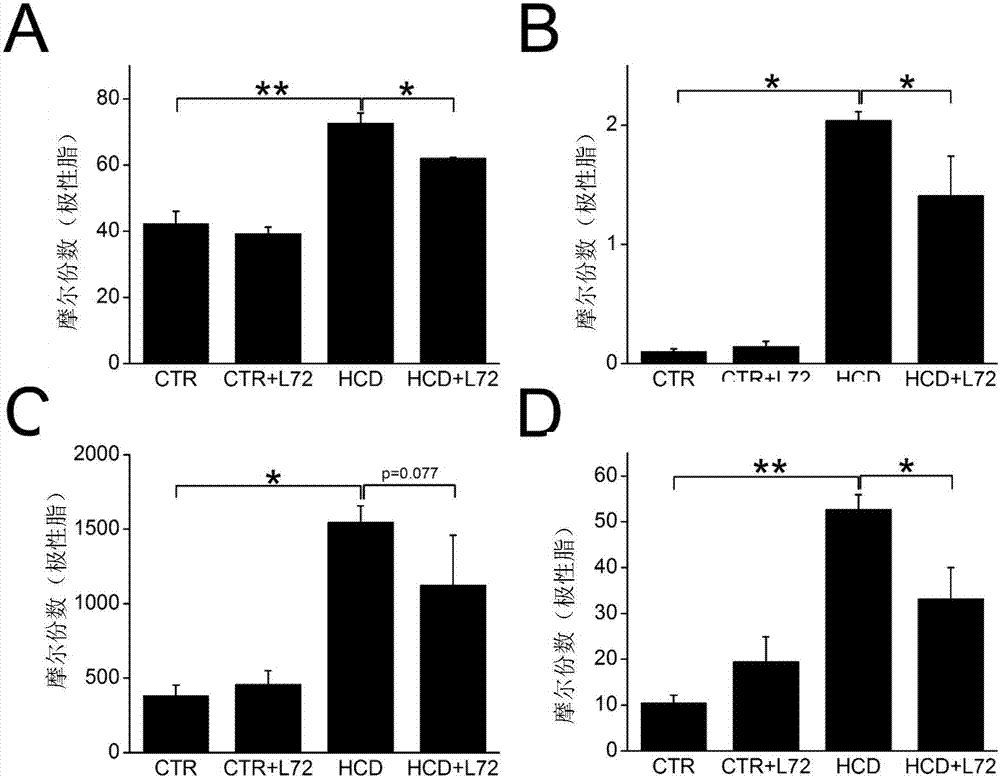
[0052] Take 17.5~23.3g, 6-8 week old male C57BL / 6 mice, feed them adaptively for 2 weeks, then divide them randomly into four groups (8 mice i...
Embodiment 2
[0071] Embodiment 2, animal test
[0072] 1. Group processing
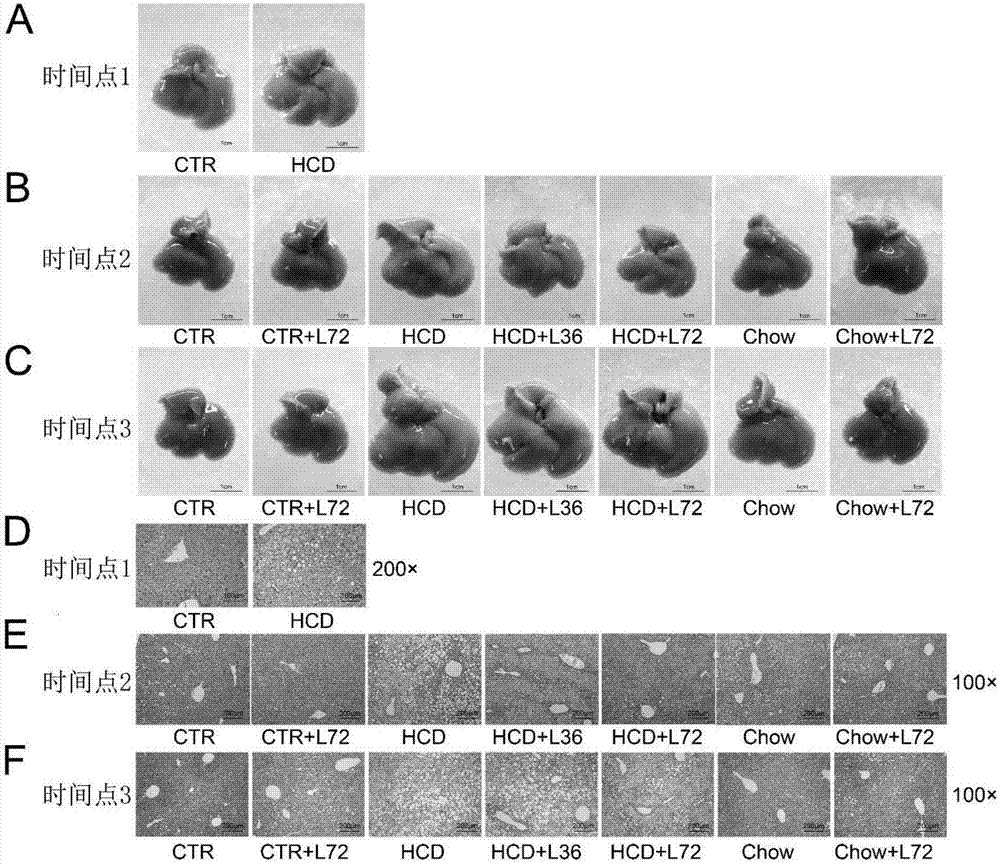
[0073] 16.7-21.3g, 6-week-old male C57BL / 6 mice were taken, fed for 2 weeks, and then tested. The test was divided into two consecutive stages.
[0074] The first stage is as follows:
[0075] The mice were randomly divided into two groups, a control group (25) and a model group (55);
[0076] Control group (CTR): fed with common feed for 18 weeks;
[0077] Model group (HCD): fed with high-cholesterol diet for 18 weeks.
[0078] After 18 weeks of the first stage (time point 1), 5 mice were randomly selected from each group to be killed, and the method in step 2 of Example 1 was used for relevant detection. The remaining mice proceeded to the second stage.
[0079] The second stage is as follows:
[0080] The remaining 20 control mice were randomly divided into two groups, a blank control group and a drug control group, with 10 mice in each group.
[0081] The remaining 50 mice in the model group were random...
Embodiment 3
[0092] Embodiment 3, cell experiment
[0093] HepG2 cells were starved for 24 hours, and then divided into three groups, which were treated as follows:
[0094] Blank control group: cultured in basal medium containing 10g / 100mL BSA for 24h;
[0095] Model group: cultured in basal medium containing 10g / 100mL BSA and 100μg / ml cholesterol for 24h;
[0096] Treatment group: cultured in basal medium containing 10 g / 100 mL BSA, 100 μg / ml cholesterol and 50 μM deferoxamine mesylate for 24 hours.
[0097] After completing the above grouping treatment, discard the culture supernatant, wash the cells with PBS buffer, then add 4% PFA solution and fix at 37°C for 10min, then soak with 60% isopropanol solution for 30s, and then use Oil Red O working solution After staining for 30 min, 60% isopropanol solution was added to wash, and then washed three times with PBS buffer, and then the staining was observed under a microscope (Nikon ECLIPSE Ti). see photos Figure 5 . Compared with the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com