Activated bifidobacteria and methods of use thereof
A bifidobacterium, composition technology, applied in the direction of bifidobacteria, application, bacteria used in food preparation, etc., can solve the problems of lack of beneficial bifidobacteria ecology, imbalance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1. Preparation of a certain bifidobacterium-specific human milk oligosaccharide (HMO) composition
[0066] A concentrated mixture of HMOs was obtained by a method similar to that described by Fournell et al. (US Patent Application 20150140175). Human milk is pasteurized and centrifuged to separate it into milkfat (mainly fat) and skim milk (skimmed product). The skim milk is then ultrafiltered using a membrane with a 5-10 kDa cut-off to concentrate the protein components (mainly whey). The permeate (comprising complex HMO) from ultrafiltration was dried by spray drying. The composition of this dry fraction is about 50% lactose and about 30% complex oligosaccharides (HMO), the remainder being mainly peptides and ash. The HMO fraction is mainly fucosylated.
[0067] This method, or a similar method, can be used to obtain compositions comprising isolated complex oligosaccharides from any mammalian milk source. For example, complex oligosaccharides can be isolat...
Embodiment 2
[0068] Example 2. Preparation of a certain bifidobacterium-specific bovine colostrum oligosaccharide (BCO) composition and a composition supplemented with synthetically produced and purified fucosylated oligosaccharide (SPF)
[0069] A concentrated mixture of bovine colostrum oligosaccharides was obtained by the method as described by Christiansen et al. (2010) International Dairy Journal, 20: 630-636. Bovine colostrum (mainly derived from the first milking) is pasteurized by heating to 145°F for 30 minutes, cooled and centrifuged to skim it, separating it into cream (mainly fat) and skim milk (skim product). The skim milk is then ultrafiltered using a membrane with a 5-10 kDa cut-off to concentrate the protein components (mainly whey). The whey permeate was further microfiltered using a membrane with a 1 kDa cut-off to remove some lactose and concentrate the oligosaccharides in the retentate. The final composition was spray dried to produce a dry oligosaccharide fraction wit...
Embodiment 3
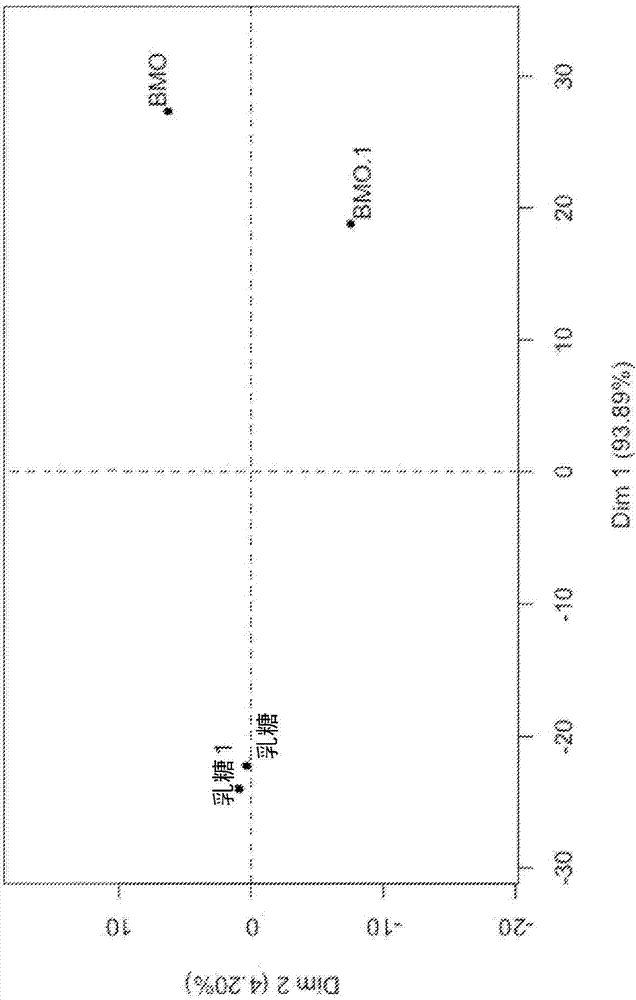
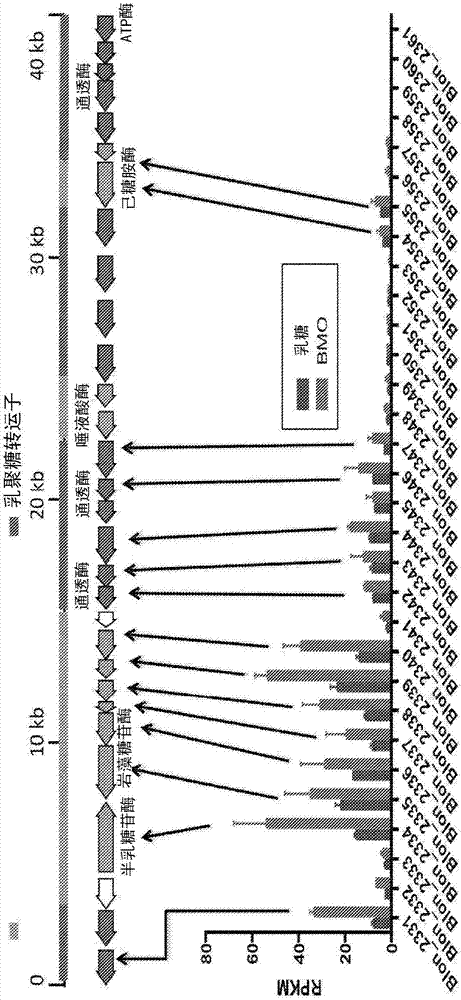
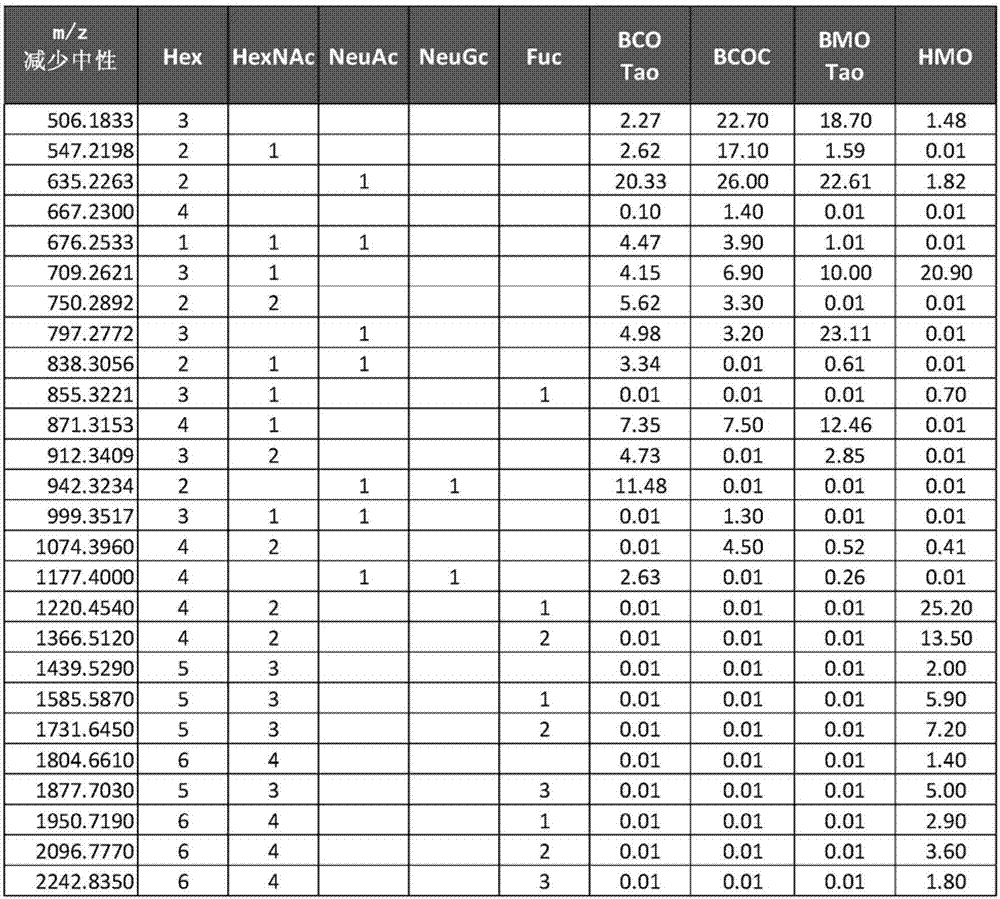
[0072] Example 3. Differences between BCO, BCOC, BMO and HMO components
[0073]BCOC fractions are commercially available as Immunel (Sterling Technology, USA) and were analyzed using the HPLC-MS method of Tao et al., (2008), J Dairy Science, 92:2991-3001 , and compared in Table 1 with oligosaccharide fractions derived from human milk (HMO), mature bovine milk (BMO) and bovine colostrum (BCO). The four compositions in Table 1 differ significantly from each other, and several features emerge immediately. BCOC contains several oligosaccharides not found in BMO, BCO or HMO, such as Hex(4) and Hex(3)HexNAc(1)NeuAc(1), and several oligosaccharides found in BCO and BMO are absent in BCOC , such as Hex(2)HexNAc(1)NeuAc(1), Hex(3)HexNAc(2), and Hex(4)NeuAc(2)NeuGc(1).
[0074]
[0075] Table 1. Key oligosaccharides distinguishing colostrum and BMO fractions as disclosed in Tao (2009), HMO fractions as revealed in Mills et al. (2012), and the bovine colostrum oligosaccharide conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com