Nitrogen-containing organoxysilane compound and method for producing same
A manufacturing method and compound technology, applied in the field of nitrogen-containing polyfunctional organooxysilane compound and its manufacturing, can solve the problems that the effect cannot be fully exerted, and achieve the effect of excellent additive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Put 204.9 g (0.6 mol) of bis(3-trimethoxysilylpropyl)amine (manufactured by Shin-Etsu Chemical Co., Ltd.), triethyl Amine 66.8g (0.66mol), heated to 80°C. After the internal temperature became stable, 63.6 g (0.32 mol) of 1,3-dibromopropane was dripped over 1 hour, and it stirred at this temperature for 20 hours. The salt produced by the reaction was removed by filtration, and the low boiling point compound was distilled off from the filtrate under heating at 90° C. / 0.4 kPa under reduced pressure to obtain 192.5 g of a liquid compound.
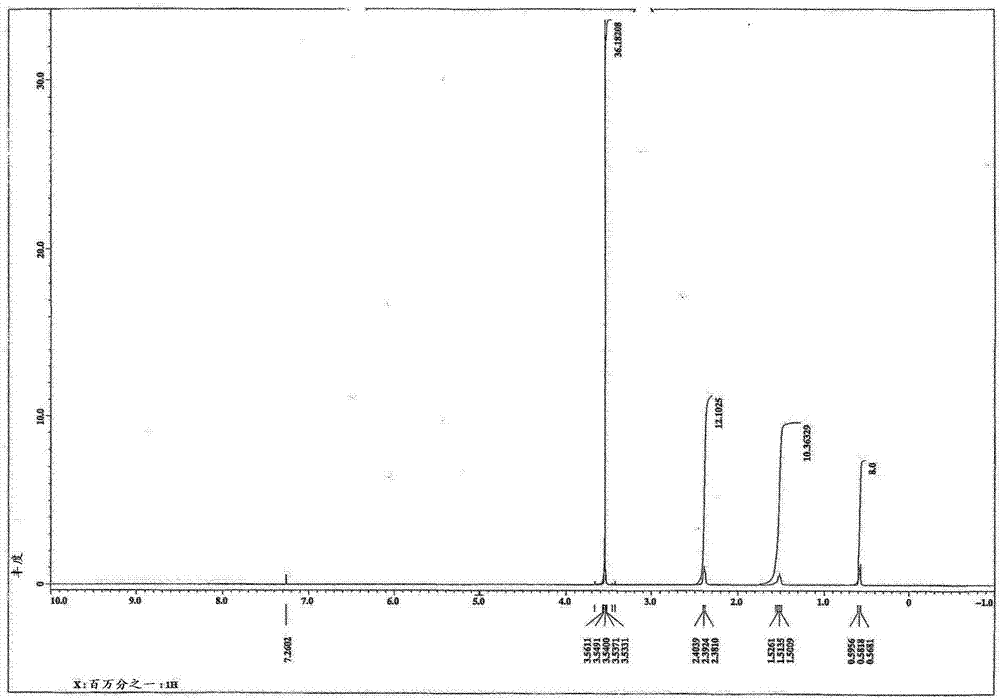
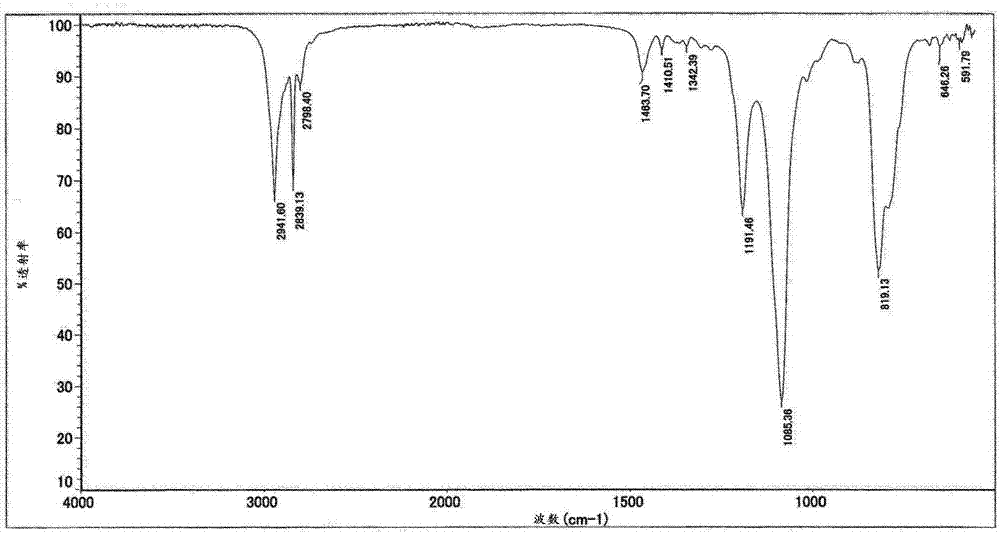
[0072] For the obtained compounds, the determination 1 H-NMR spectrum (deuterated chloroform solvent) and IR spectrum. The results are shown in image 3 , 4 in.
[0073] From the above results, it was confirmed that the obtained compound was N,N,N',N'-tetrakis(3-trimethoxysilylpropyl)-1,3-propanediamine.
Embodiment 2
[0075] Put [3-dimethoxy(4-pentenyloxy)silylpropyl]-(3-trimethoxysilylpropyl) into a flask equipped with stirrer, reflux, dropping funnel and thermometer 197.9 g (0.5 mol) of amine (manufactured by Shin-Etsu Chemical Co., Ltd.) and 71.1 g (0.55 mol) of ethyldiisopropylamine were heated to 80°C. After the internal temperature became stable, 53.0 g (0.27 mol) of 1,3-dibromopropane was dripped over 1 hour, and it stirred at this temperature for 20 hours. The salt produced by the reaction was removed by filtration, and the low boiling point compound was distilled off from the filtrate under heating at 90° C. / 0.4 kPa under reduced pressure to obtain 204.9 g of a liquid compound.
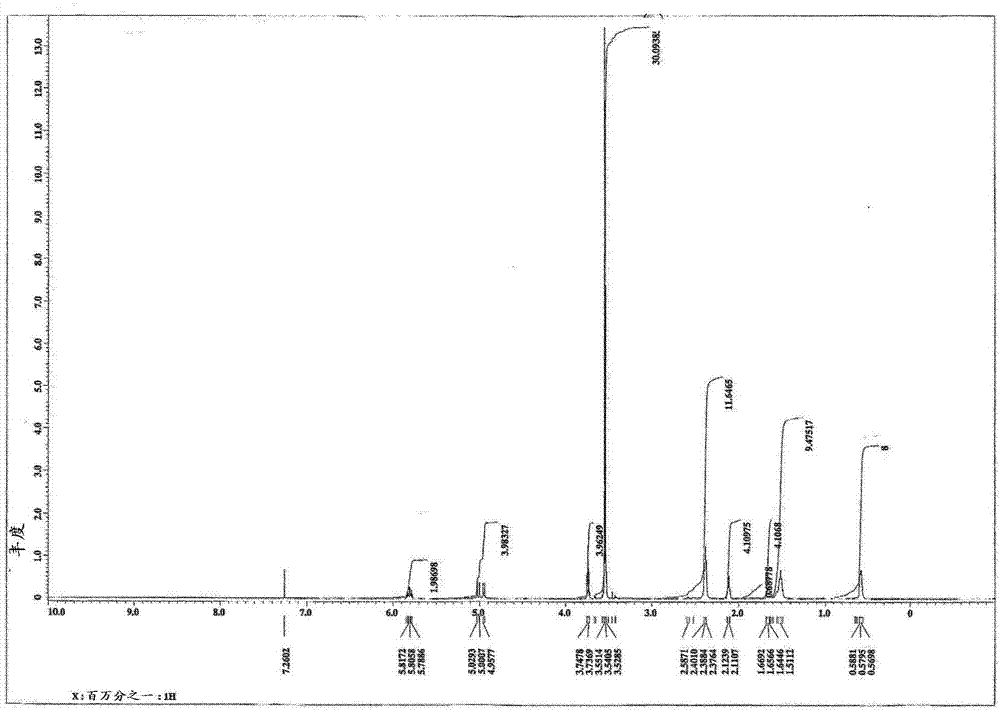
[0076] For the obtained compounds, the determination 1 H-NMR spectrum (deuterated chloroform solvent) and IR spectrum. The results are shown in image 3 , 4 in.
[0077] From the above results, it was confirmed that the obtained compound was N,N'-bis[3-dimethoxy(4-pentenyloxy)silylpropyl]-N,N'-bis(3-tr...
Embodiment 3
[0079] Put [3-dimethoxy(1-methyl-2-methoxyethoxy)silylpropyl]-(3- 199.9 g (0.5 mol) of trimethoxysilylpropyl)amine (manufactured by Shin-Etsu Chemical Co., Ltd.), and 55.7 g (0.55 mol) of triethylamine were heated to 80°C. After the internal temperature became stable, 53.0 g (0.27 mol) of 1,3-dibromopropane was dripped over 1 hour, and it stirred at this temperature for 20 hours. The salt produced by the reaction was removed by filtration, and the low boiling point compound was distilled off from the filtrate under heating at 90° C. / 0.4 kPa under reduced pressure to obtain 204.0 g of a liquid compound.
[0080] For the obtained compounds, the determination 1 H-NMR spectrum (deuterated chloroform solvent) and IR spectrum. The results are shown in Figure 5 ,6 in.
[0081] From the above results, it was confirmed that the obtained compound was N,N'-bis[3-dimethoxy(1-methyl-2-methoxyethoxy)silylpropyl]-N,N'-bis (3-Trimethoxysilylpropyl)-1,3-propanediamine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com