Heteroatom-doped flowerene derivatives and synthesis method thereof
A technology for hydrinoflorene and heteroatom, which is applied in the field of heteroatom-doped florene derivatives and the preparation thereof, can solve the problems of low total yield, difficult synthesis of raw materials, inability to synthesize parent structures, etc., and achieves short synthesis steps, Easy bulk preparation, mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
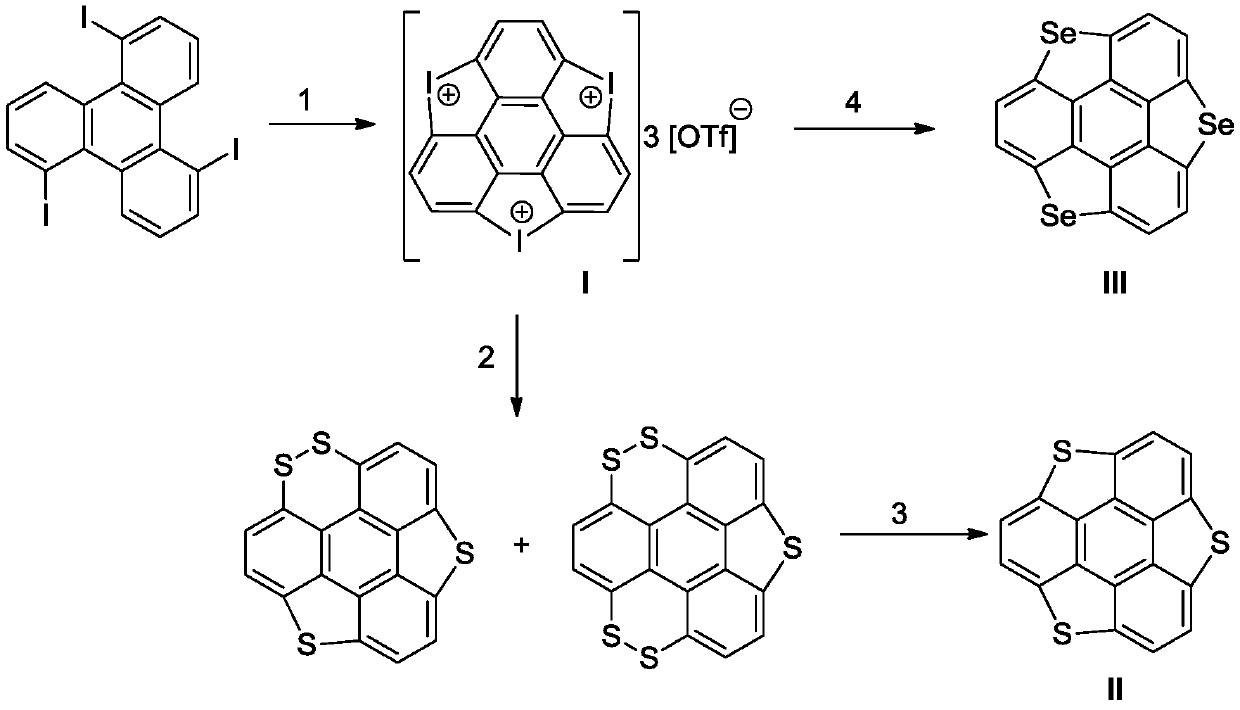
[0034] Embodiment 1: Taking the synthesis of iodofloralene compound as an example, its structural formula is as follows:
[0035]
[0036] Dissolve 2.18g of 1,5,9-triiodotriphenylene (refer to patent for preparation method: 1,5,9-trisubstituted ketone compound and its synthesis method, application number: 201610962972.8) in 100mL of dichloromethane In the flask, 3.6g of m-chloroperoxybenzoic acid (70%wt%) in dichloromethane (100mL) and 1.9mL of trifluoromethanesulfonic acid were added dropwise at room temperature, reacted for 12h at 20°C, and the reaction was completed Finally, remove the solvent, add diethyl ether, filter with suction, wash with diethyl ether, the obtained brown solid is 2.73g of iodofloralene salt compound, the yield is 72%, melting point: >300°C.
[0037] The characterization of the gained iodine salt compound is as follows: IR (KBr, cm -1 ):3083,1701,1642,1549,1370,1241,1176,1029,809,641; 1 H NMR (500MHz, DMSO-d 6 ):δ8.77(s,6H); 19 F NMR (470MHz, DM...
Embodiment 2
[0038] Embodiment 2: Taking the synthesis of thiaflorene as an example, its structural formula is as follows:
[0039]
[0040] Raw materials used and their synthesis methods:
[0041] 1. Synthesis of thia polycyclic aromatic compounds
[0042] Method A: Add 525mg of the iodine salt compound in Example 1 together with 96mg of sulfur powder and 1.6g of cesium carbonate into a Schlenk tube filled with 10mL of dimethyl sulfoxide, under the protection of an inert gas, at 140°C React for 12 hours. After the reaction is completed, add water, then add dichloromethane for extraction, wash the organic phase with water, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate the solvent, add ethanol, and filter with suction. The obtained brown solid is a thia polycyclic aromatic Compound 87.5mg, its yield 50%, melting point: >300°C.
[0043] Method B: 525mg of the iodine salt compound in Example 1 was added together with 6.7mg of copper chloride and 343mg of potas...
Embodiment 3
[0046] Embodiment 3: Taking the synthesis of thiaflorene as an example, its structural formula is as follows:
[0047]
[0048] Add 315mg of the iodine salt compound in Example 1 together with 142mg of selenium powder and 336mg of potassium tert-butoxide into a Schlenk tube containing 8mL of dimethyl sulfoxide, and react at 70°C for 12h under the protection of an inert gas , after the reaction is complete, add water, then add dichloromethane for extraction, wash the organic phase with water, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate the solvent, add ethanol, and filter with suction, the resulting brown solid is mixed with 1.5g of copper powder and 4mL of 1 , 2,3,4-Tetrahydronaphthalene was added to the Schlenk tube together, and reacted at 200°C for 1 hour. After the reaction was completed and cooled, dichloromethane was added, filtered with suction, the filtrate was desolventized, n-hexane was added, and the obtained brown-yellow solid It is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com