Improved method of pomalidomide synthesis process
A technique for the synthesis of pomalidomide, which is applied in the field of preparation of pomalidomide, and can solve problems such as large environmental pollution, long process routes, and solvent residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
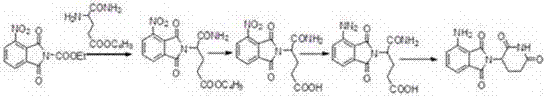
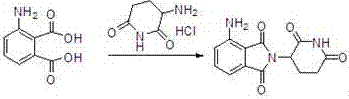
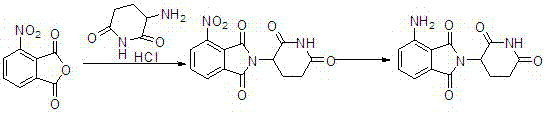
[0030] Pump N,N-dimethylformamide (50kg) into the reactor, add glutamic acid (32kg) and 3-nitrophthalic anhydride (40kg) under stirring, then heat, at 95-100 ℃ for 3 hours, then distill N,N-dimethylformamide under reduced pressure, add acetic anhydride (50kg) under cooling, heat to 100-110℃ for 1 hour, distill off excess anhydride under reduced pressure, add DMSO (400L) , heat the reaction to 200°C, and slowly introduce ammonia gas under stirring. After the reaction, cool to room temperature and add methanol (800L), crystallize and centrifuge, and dry to obtain 3-nitropomalidomide (62.76kg) , yield 71%. 99.99% purity.
[0031] 3-Nitropomalidomide (30kg) was dissolved in 1,4-dioxane\methanol=300L\300L, added 10% palladium carbon, 35°C, 0.4M, hydrogenated until the reaction was complete, press-filtered, and reduced Concentrate to dryness under pressure, add DMF (300L) to dissolve, crystallize from isopropyl ether (600L), centrifuge to obtain a yellow product, and then use etha...
Embodiment 2
[0033] Pump pyridine (50kg) into the reactor, add glutamic acid (32kg) and 3-nitrophthalic anhydride (40kg) under stirring, then heat, react at 95-100°C for 5 hours, and then depressurize Evaporate pyridine, cool and add acetic anhydride (50kg), heat to 100-110°C to react for 2 hours, evaporate excess anhydride under reduced pressure, add DMSO (400L), heat the reaction to 180°C, and slowly introduce ammonia under stirring After the reaction, cool to room temperature, add ethanol (800L) to crystallize and centrifuge, and dry to obtain 3-nitropomalidomide (62.76kg), with a yield of 71% and a purity of 99.2%.
[0034] 3-Nitropomalidomide (30kg) was dissolved in 1,4-dioxane\methanol=150L\150L, added 10% palladium carbon, 35°C, 0.4Mp, hydrogenated until the reaction was complete, press-filtered, reduced Concentrate to dryness under pressure, add DMF (300L) to dissolve, crystallize from isopropyl ether (600L), centrifuge to obtain a yellow product, and then use ethanol to recrysta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com