Synthesis method of 3-arylisoindol derivatives
A technology for aryl isoindolinone and its derivatives, which is applied in the field of synthesis technology for preparing 3-aryl isoindolinone, can solve the problems of harsh conditions and low yield, and achieve good reaction universality, Easily extendable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
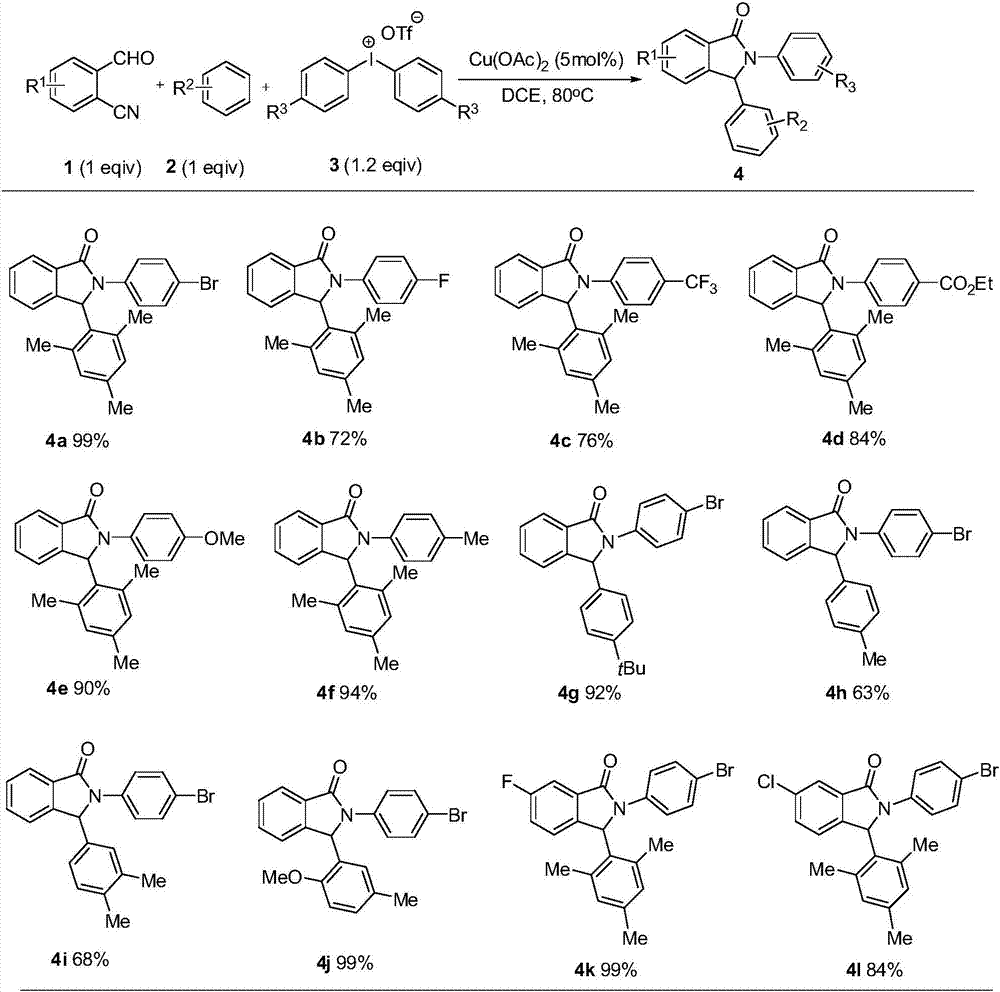
[0016] O-cyanobenzaldehyde (1mmol), 1,3,5-trimethylbenzene (1mmol), bis(4-bromophenyl)iodonium trifluoromethanesulfonate (1.2mmol), copper acetate (5mmol%) and 2mL Add o-dichloroethane into a 15mL pressure-resistant tube, stir at 110°C, react for 2.5 hours, and separate by silica gel column chromatography to obtain 4a with a yield of 99%. 1 H NMR (300MHz, CDCl 3 )δ7.94-7.92(m,1H),7.48-7.43(m,4H),7.37-7.34(m,2H),7.16-7.13(m,1H),6.93(s,1H),6.57(s, 1H),6.44(s,1H),2.66(s,3H),2.18(s,3H),1.61(s,3H). 13 C NMR (75MHz, CDCl 3 )δ167.8, 143.8, 138.1, 136.9, 132.8, 131.8, 129.9, 129.1, 128.5, 123.9, 122.9, 122.4, 117.8, 61.4, 21.3, 20.8, 18.9.
Embodiment 2
[0018] O-cyanobenzaldehyde (1mmol), 1,3,5-trimethylbenzene (1mmol), bis(4-fluorophenyl)iodonium trifluoromethanesulfonate (1.2mmol), copper acetate (5mmol%) and 2mL Add o-dichloroethane into a 15mL pressure-resistant tube, stir at 110°C, react for 2.5 hours, and separate by silica gel column chromatography to obtain 4b with a yield of 72%. 1 H NMR (300MHz, CDCl 3 )δ7.97-7.94(m,1H),7.51-7.44(m,4H),7.18-7.15(m,1H), 6.99-6.91(m,3H),6.60(s,1H),6.47(s, 1H), 2.62(s,3H), 2.19(s,3H), 1.64(s,3H). 13 C NMR (75MHz, CDCl 3 )δ167.9, 159.9 (d, J = 242.8Hz), 144.1, 138.0, 136.9 (d, J = 18.7Hz), 133.8, 132.6, 131.7, 129.8, 128.5, 123.8 (d, J = 7.5Hz), 122.4, 115.7 (d, J=22.5Hz), 61.9, 21.3, 20.8, 18.9. 19 F NMR (282MHz, CDCl 3 )δ-116.7.
Embodiment 3
[0020] O-cyanobenzaldehyde (1mmol), 1,3,5-trimethylbenzene (1mmol), bis(4-trifluoromethylbenzene)iodonium trifluoromethanesulfonate (1.2mmol), copper acetate (5mmol%) ) and 2 mL of o-dichloroethane were added to a 15 mL pressure-resistant tube, stirred at 110° C., reacted for 2.5 hours, and separated by silica gel column chromatography to obtain 4c with a yield of 76%. 1 H NMR (300MHz, CDCl 3 )δ7.98-7.95(m,1H),7.74-7.71(m,2H),7.55-7.52(m,4H),7.19-7.17(m,1H),6.96(s,1H),6.59-6.54( m,2H),2.72(s,3H),2.21(s,3H),1.63(s,3H). 13 C NMR (75MHz, CDCl 3 )δ168.2, 143.7, 138.2, 136.8, 136.2, 133.1, 131.9, 129.9, 128.7, 126.0, 124.0, 122.5, 120.6, 61.4, 21.3, 20.8, 18.9. 19 F NMR (282MHz, CDCl 3 )δ-62.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com