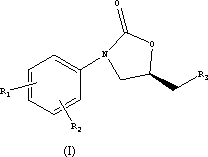
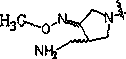
Novel oxazolidinone compound as well as preparation method and medical application thereof
A technology of oxazolidinones and compounds, applied in chemical instruments and methods, active ingredients of heterocyclic compounds, compounds of Group 5/15 elements of the periodic table, etc., can solve the problem that the antibacterial spectrum cannot fully cover upper respiratory tract infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: N-(((S)-3-(3-fluoro-4-((4aR,7aR)-octahydropyrrolo[3,4-b]pyridin-6-yl)phenyl)-2 Preparation of -oxooxazolidin-5-yl)methyl)acetamide (Compound A)
[0037]
[0038] Compound A
[0039] Take (4aR,7aR)-6-(4-bromo-2-fluorophenyl)-octahydro-1H-pyrrolo[3,4-b]pyridine 10g, add 200ml of ethyl acetate to dissolve, and lower the temperature of the solution to below 5°C, then add 6.5g of N-(((S)-2-oxooxazolidin-5-yl)methyl)acetamide in batches, control the internal temperature not to exceed 10°C, and let the bath The temperature was naturally raised to room temperature, and the reaction was stirred for 8 hours, then the insoluble matter was filtered off, washed with a small amount of chloroform, combined with the chloroform solution, washed twice with water, dried over anhydrous sodium sulfate, and concentrated to dryness. The residue was separated by HPLC to obtain 3.5 g of compound A.
Embodiment 2
[0040] Example 2: (S)-3-(3-fluoro-4-((4aR,7aR)-octahydropyrrolo[3,4-b]pyridin-6-yl)phenyl)-5-((form Preparation of (amino)methyl)oxazolidin2-one (compound B)
[0041]
[0042] Compound B
[0043] Prepared according to the method of Example 1, except that N-(((S)-2-oxooxazolidin-5-yl)methyl)acetamide is replaced by (S)-5-((dimethyl amino)methyl)oxazolidin-2-one.
Embodiment 3
[0044] Example 3: N-(((S)-3-(4-((R)-3-aminoazepan-1-yl)-3-fluorophenyl)-2-oxooxazolidine Preparation of -5-yl)methyl)acetamide (Compound C)
[0045]
[0046] Compound C
[0047]Take 10g of (R)-1-(4-bromo-2-fluorophenyl)azepan-3-amine, add 200ml of dichloromethane to dissolve, lower the temperature of the solution to below 5°C, and then add in batches N-(((S)-2-oxooxazolidin-5-yl)methyl)acetamide 7.5g, control the internal temperature not to exceed 10°C, after the addition, let the bath temperature naturally rise to room temperature, and stir the reaction 8h, then filter out the insoluble matter, wash with a small amount of chloroform and combine the chloroform solution, then wash twice with water, dry over anhydrous sodium sulfate, and concentrate to dryness. The residue was separated by HPLC to obtain 4.2 g of compound C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com