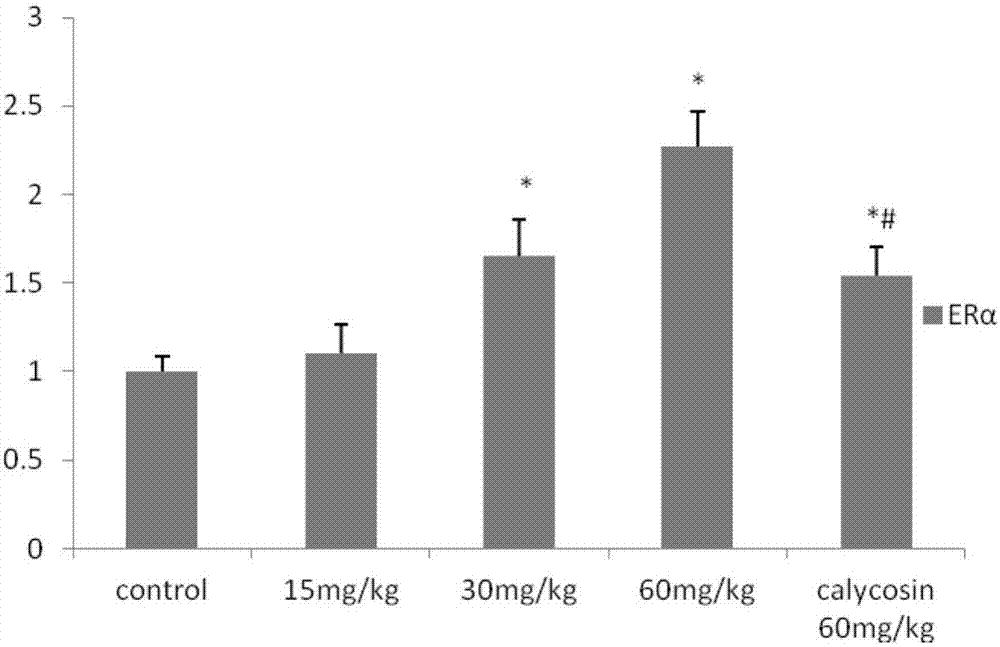
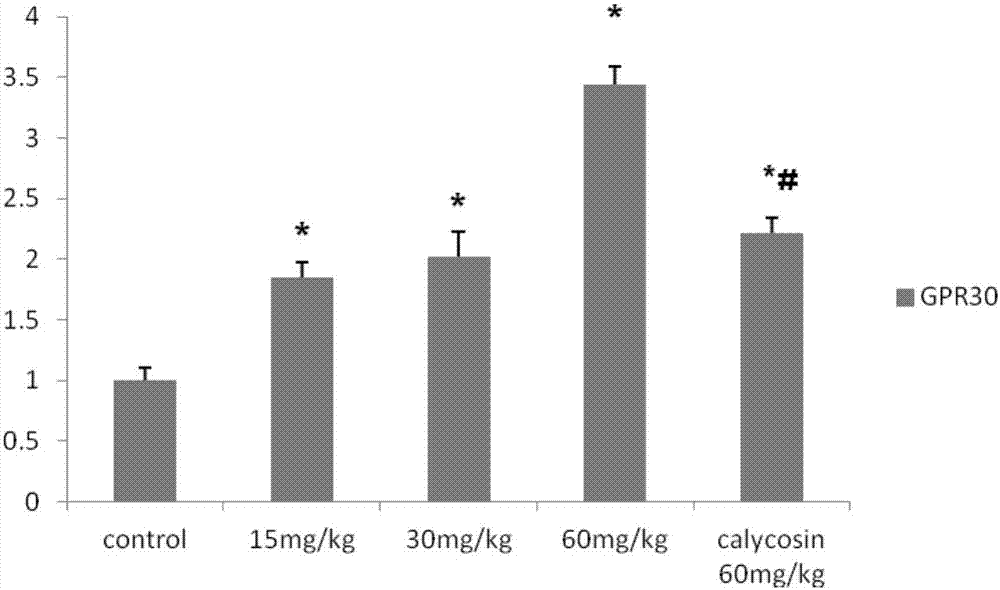
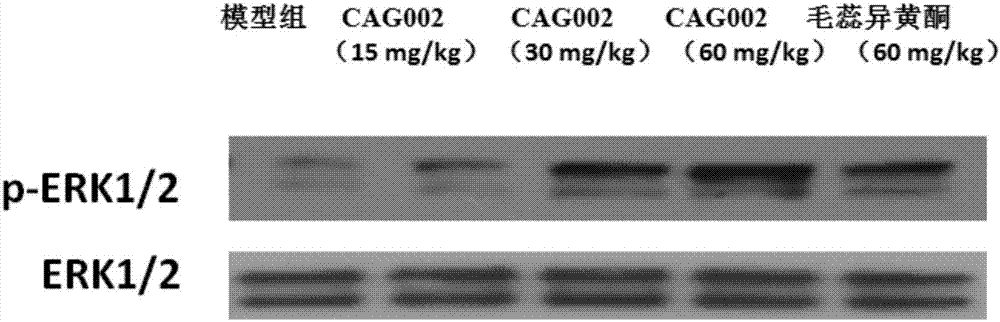
Application of calycosin derivative in preparing medicine for treating cerebral ischemia reperfusion injury
A technology of cerebral ischemia reperfusion and drugs, applied in the field of medicine, can solve problems such as retention, inconsistency, and lack of targets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the synthesis of the compound shown in the formula (I) used in the following each experimental examples of the application:
[0031] Synthesized according to the following synthetic route:
[0032]
[0033] Among them, the chemical name of compound 1 is: 7-hydroxy-3-(3-hydroxy-4-methoxy)-4H-benzopyran-4-one, and the chemical name of compound 2 is: 1,2-di Bromoethane, the chemical name of intermediate product 3 is: 7-(2-bromoethoxy)-3-(3-(2-bromoethoxy)-4-methoxyphenyl)-4H-benzo Pyran-4-one, the chemical name of compound 4 is: morpholine; the chemical name of compound 5 is: 3-(4-methoxy-3-(2-morpholinoethoxy)phenyl)-7 -(2-Morpholinoethoxy)-4H-benzopyran-4-one.
[0034] The specific synthesis method is:
[0035]1) Take 7-hydroxy-3-(3-hydroxy-4-methoxy)-4H-benzopyran-4-one (300mg) and 1,2-dibromoethane in a molar ratio of 1:8 Dissolve in 13mL of acetone, then use triethylamine to adjust the pH of the system to 10, react at room temperature for 8h, spin...
experiment example 1
[0047] Experimental example 1: neurological function score
[0048] After postoperative anesthesia, the behavioral deficits of the animals were scored according to the method of Bederson. 0 points: no neurological symptoms were observed; 1 point: when the tail was suspended in the air, the contralateral forelimb of the animal showed wrist and elbow flexion, elbow abduction, and was close to the chest wall; 2 points: the animal was placed on a smooth surface, and the operated side was pushed When the shoulder moves to the opposite side, the resistance decreases; 3 points: the animal walks around or circles to the opposite side of the operation; 4 points: flaccid paralysis, no spontaneous movement of the limbs. The higher the score, the more severe the behavioral disorder. Weed out unsuccessful models
[0049] The results showed that CAG002 can dose-dependently reduce the cerebral ischemia-reperfusion injury score in rats. The effect is most obvious when the drug concentratio...
experiment example 2
[0053] Experimental example 2: TCC detection of cerebral infarction area
[0054] After 2 hours of cerebral ischemia and 24 hours of reperfusion, the brain was decapitated and taken out. The brain was sliced into 2 mm coronal slices, totaling 5 slices. Then the slices were placed in 2% TTC phosphate buffer and incubated at a constant temperature of 37°C for 30 min. Normal brain tissue was stained as Red, with white infarcts. Calculate the mass percentage of the infarcted part in the brain slice to reflect the infarct volume ratio.
[0055] The results showed that CAG002 could dose-dependently reduce the volume of cerebral infarction in rats with cerebral ischemia-reperfusion injury. Among them, when the drug concentration is 60mg / kg, the effect is the most obvious. Compared with the positive group, CAG002 is also significantly better than the calycosin group (p<0.05), as shown in Table 2.
[0056] Table 2 Effects of different concentrations of CAG002 and calycosin on cereb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com