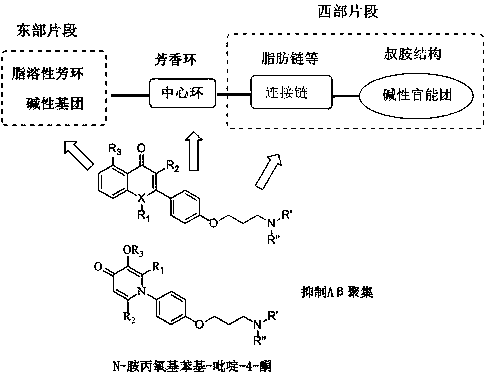
Preparation and applications of phenylquinolinone-based and flavone-based derivative
A technology of benzoquinolinone and benzoquinolinone thiazine, which is applied in the field of preparation of phenylquinolinone and flavonoid derivatives, and can solve the problems of nerve cell toxicity and nerve cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Synthesis of I-1 and I-2 in class I compounds (quinolinones).
[0066] Step 1: 4-(3-chloropropoxy)benzoic acid (Intermediate 1).
[0067] Ethyl p-hydroxybenzoate II-1 (12 g, 72 mmol), 1,3-bromochloropropane (14.2 mL, 144 mmol) and K 2 CO 3 (20 g, 144 mmol) was dissolved in 100 mL of acetonitrile and heated to reflux for 12 h. Suction filtration to remove excess K 2 CO 3 , the solvent was distilled off under reduced pressure to obtain a colorless oily liquid. Directly add 15 mL of 6 N NaOH solution and 30 mL of CH 3 OH, reflux for 1 h. After the reaction solution became clear, it was cooled and acidified with 2 N hydrochloric acid to pH = 2. A large amount of white solid was precipitated, filtered with suction, washed with water, and dried to obtain 14.5 g of white solid powder. Yield 94%; 1 H NMR (500 MHz, CDCl 3 ): δ 8.08 (d, J = 8.5 Hz, 2H), 6.96 (d, J = 8.5 Hz, 2H), 4.21 (t, J = 6.0 Hz, 2H), 3.77 (t, J = 6.5 Hz, 2H),2.30-2.25 (m, 2H); ESI-...
Embodiment 2
[0076] Example 2: Synthesis of I-3~I-6 in class I compounds (quinolinones).
[0077] Step 1: 2-(4-(3-chloropropoxy)phenyl)-1-methylquinoline-4(1 H )-ketone (intermediate 5a).
[0078] Intermediate 4 (276 mg, 0.88 mmol) was dissolved in 5 mL DMF, 42 mg NaH (60%, 1.05 mmol) was added, stirred at room temperature for 30 min, then methyl iodide (138 mg, 0.97 mmol) was added, and the temperature was raised To 35°C, react for 30min. Pour the reaction solution into 50 mL H 2 O, extracted with EtOAc, washed with water, washed with saturated NaCl, anhydrous NaCl 2 SO 4 dry. After recovering the solvent, it was separated by column chromatography (petroleum ether: EtOAc=10: 1) to obtain 250 mg of white solid. Yield 87%; 1 H NMR (500MHz, CDCl 3 ): δ 8.17 (d, J = 8.0 Hz, 1H), 8.10-8.08 (m, 3H), 7.71 (d, J = 8.0,1H), 7.48 (t, J = 7.5 Hz, 1H), 7.14 (s, 1H), 7.05 (d, J = 9.0 Hz, 2H), 4.21 (t, J = 6.0 Hz, 2H), 4.12 (s, 3H), 3.79 (t, J = 6.5 Hz, 2H), 2.31-2.26 (m, 2H); ESI-...
Embodiment 3
[0089] Example 3: Synthesis of I-7 and I-8 in class I compounds (quinolinones).
[0090] Step 1: 4-(3-chloropropoxy)acetophenone (intermediate 6).
[0091] p-Hydroxyacetophenone (5.0 g, 36.7 mmol), 1,3-bromochloropropane (7.3 mL, 73.5 mmol) and K 2 CO 3 (10 g, 73.5 mmol) was dissolved in 30 mL of acetonitrile, heated to reflux for 10 h. Suction filtration to remove excess K 2 CO 3 , the solvent was distilled off under reduced pressure, and separated by column chromatography (petroleum ether: EtOAc=10: 1) to obtain 7.6 g of a colorless liquid. Yield 98%; 1 H NMR (500MHz, CDCl 3 ): δ 7.95 (d, J = 9.0 Hz, 2H), 6.95 (d, J = 9.0 Hz, 2H), 4.20 (t, J =6.0 Hz, 2H), 3.77 (t, J = 6.0 Hz, 2H), 2.56 (s, 3H), 2.29-2.24 (m, 2H); ESI-MS: m / z =213 [M+H] + .
[0092] Step 2, 2-bromo-1-(4-(3-chloropropoxy)phenyl)ethanone (intermediate 7).
[0093] Intermediate 6 (2.12 g, 10 mmol) was dissolved in 20 mL of ether, and Br was slowly added dropwise at 0°C 2 (0.51 mL, 10 mmol), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com