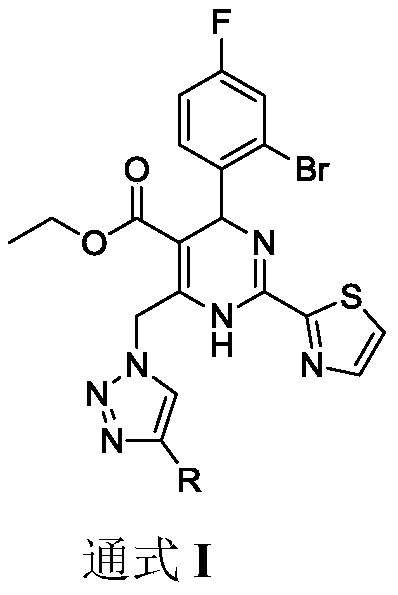
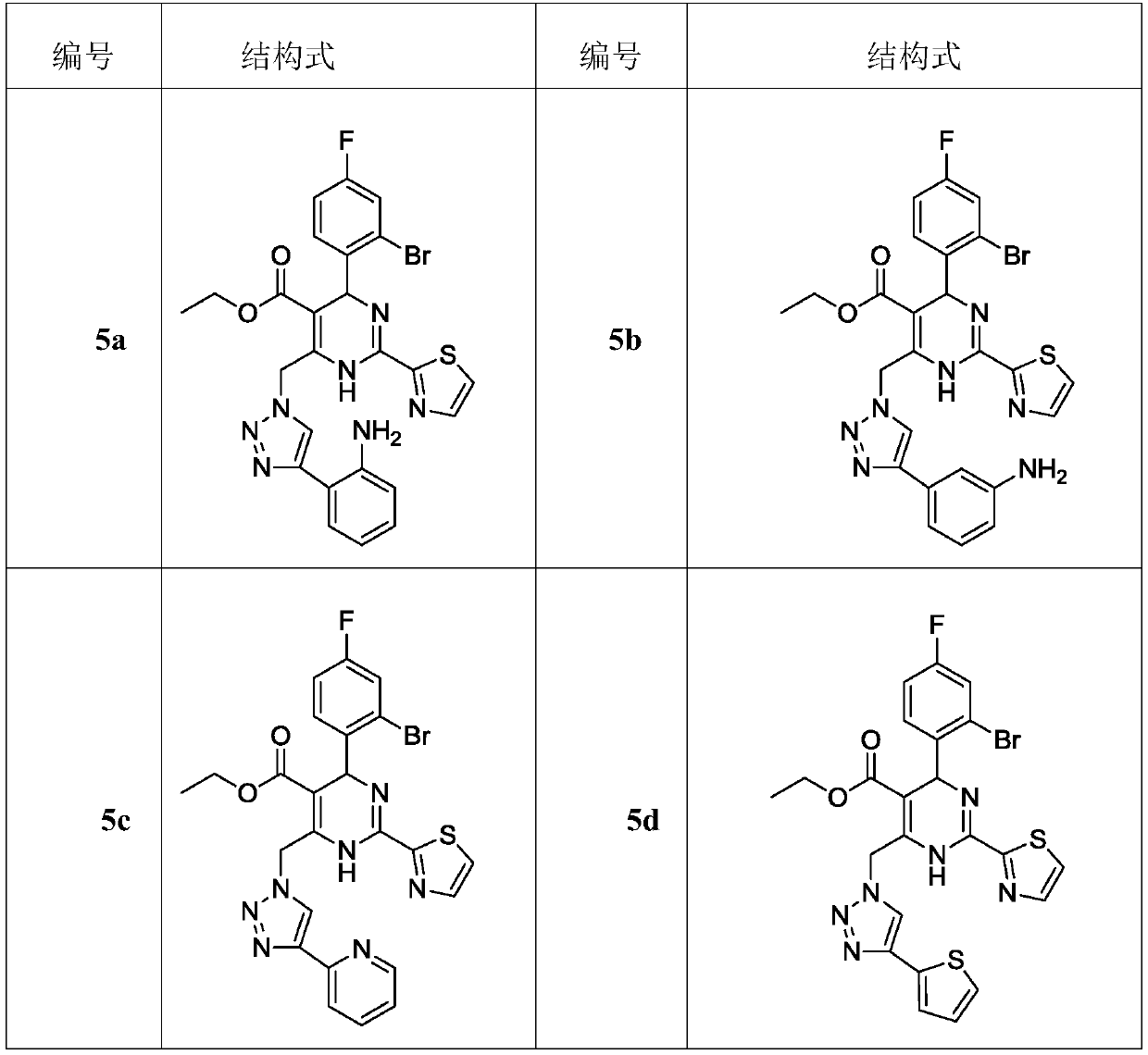
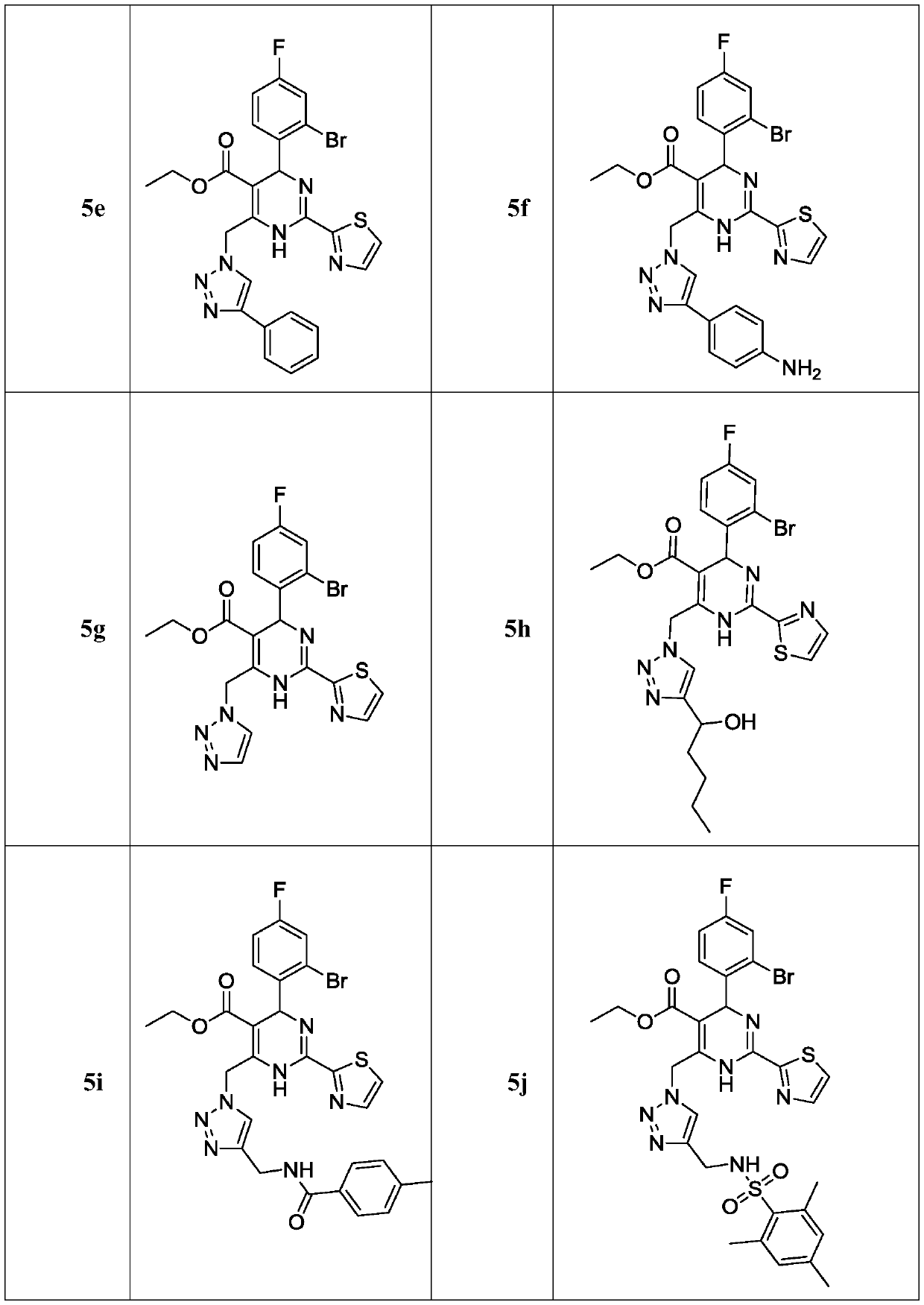
Dihydropyrimidine-triazole derivatives, preparation method and application thereof
A technology of triazoles and dihydropyrimidines, applied in the field of medicine, can solve problems such as poor water solubility, strong liver toxicity, and poor metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1. Preparation of Compound 2
[0042] Take a 500mL round-bottomed flask, dissolve 2-thiazolecarboxamidine hydrochloride (2.00g, 12.22mmol) in 250mL absolute ethanol, and add 2-bromo-4-fluorobenzaldehyde (3.74g, 18.42mmol) successively at room temperature ), ethyl acetoacetate (1559μL, 12.22mmol), sodium acetate (1.66g, 12.22mmol), reflux at 80°C for 6h. After the reaction, cool to room temperature, remove absolute ethanol by rotary evaporation, add water (60mL), extract with ethyl acetate three times (25mL x 3), combine organic phases, wash with saturated brine three times (30mL x 3), anhydrous sodium sulfate Drying; concentration, dry loading, flash preparative chromatography on a silica gel column, recrystallization from a dichloromethane-n-hexane mixed solvent to obtain 3.98 g of a yellow solid with a yield of 76%; melting point 153-156°C.
[0043]
[0044]Compound 2 spectral data: 1 H NMR (400MHz, CDCl 3 )δ7.81(d, J=2.8Hz, 1H), 7.46(s, 1H), 7.38–7.2...
Embodiment 2
[0045] Embodiment 2. Preparation of compound 3
[0046] Take a 500mL round bottom flask, dissolve Intermediate 2 (2.00g, 4.71mmol) in 200mL carbon tetrachloride, slowly add NBS (0.88g, 4.94mmol), and react at reflux at 50°C for 10h. After the reaction, cool to room temperature, remove carbon tetrachloride by rotary evaporation, add water (50mL), extract with ethyl acetate three times (20mL x 3), combine organic phases, wash with saturated brine three times (25mL x 3), anhydrous sulfuric acid Drying over sodium; concentration, dry loading, flash preparative chromatography on a silica gel column, recrystallization from a dichloromethane-n-hexane mixed solvent to obtain 1.21 g of a yellow solid with a yield of 51%; melting point 123-128°C.
[0047]
[0048] Spectral data of compound 1: 1 H NMR (400MHz, CDCl 3 )δ7.84(d,J=3.1Hz,1H),7.52(s,2H),7.44–7.35(m,1H),7.32(dd,J=8.1,2.6Hz,1H),7.02(t,J =8.0Hz,1H),6.09(s,1H),4.94(d,J=8.9Hz,1H),4.61(s,1H),4.09(d,J=7.0Hz,2H),1.16(t,J =7.1H...
Embodiment 3
[0049] Embodiment 3. Preparation of compound 4
[0050] Take a 100mL round bottom flask, dissolve intermediate X-3 (0.89g, 1.77mmol) in 45mL acetone, add NaN 3 (0.23g, 3.54mmol), stirred overnight at room temperature. After the reaction, cool to room temperature, remove carbon tetrachloride by rotary evaporation, add water (50mL), extract with ethyl acetate three times (20mL x 3), combine organic phases, wash with saturated brine three times (25mL x3), anhydrous sodium sulfate Drying; concentration, dry loading, flash preparative chromatography on a silica gel column, recrystallization from a dichloromethane-n-hexane mixed solvent to obtain 0.85 g of a yellow solid, yield 91%; melting point 123-126°C.
[0051]
[0052] Compound 4 spectral analysis data: 1 H NMR (400MHz, CDCl 3 )δ8.64(s,1H),7.85(d,J=3.1Hz,1H),7.55(d,J=3.1Hz,1H),7.48–7.37(m,1H),7.35–7.29(m,1H ),7.10–6.92(m,1H),6.29–6.02(m,1H),4.97(s,1H),4.60(d,J=2.6Hz,1H),4.17–4.00(m,2H),1.13( t,J=7.1Hz,3H); 13 C NMR (1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com