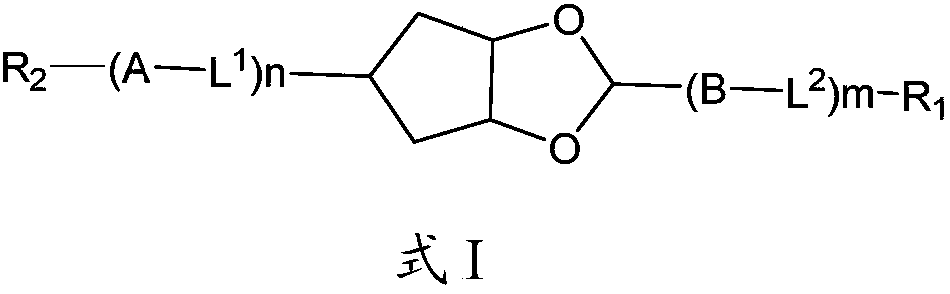
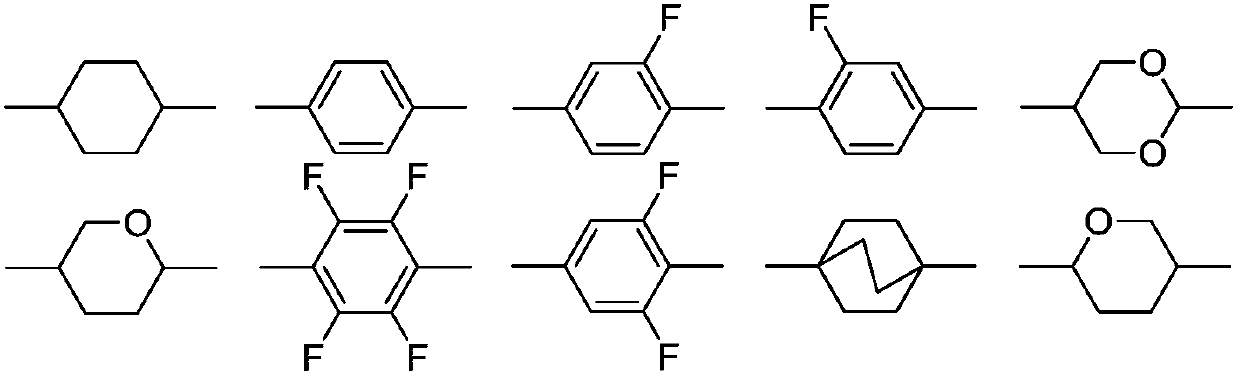
Compounds, liquid crystal composition and liquid crystal display
A technology of liquid crystal composition and compound, which is applied in the direction of liquid crystal materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of low clearing point and the inability of liquid crystal displays to be used at high temperatures, so as to increase the working temperature range and improve The effect of clearing the spot and increasing the temperature range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
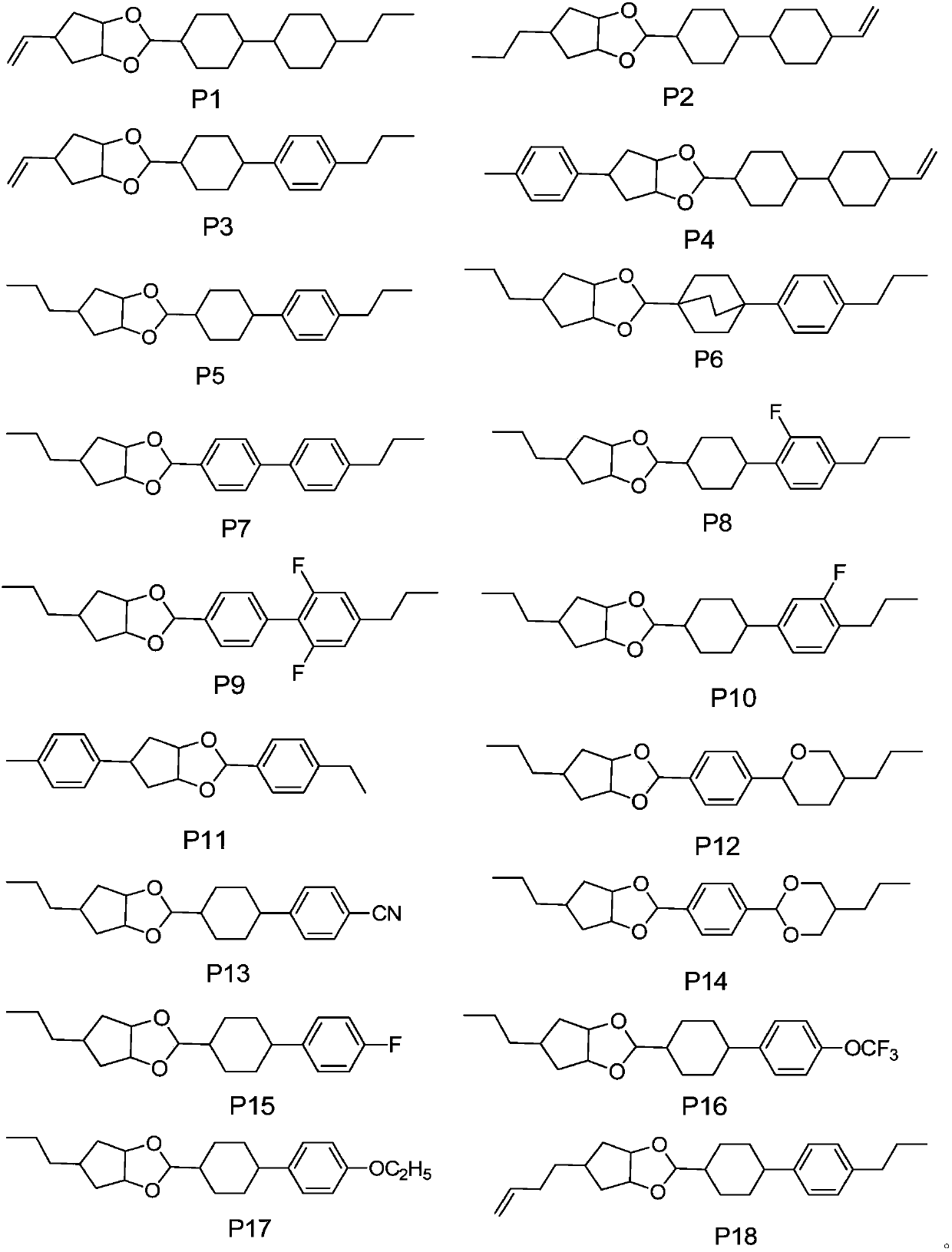
[0063] The specific compound synthesized is shown in formula P1:
[0064]
[0065] The synthetic route of P1 is as follows:
[0066]
[0067] That is, the compound 3,4-dihydroxy-1-vinyl cyclopentane and propyl biscyclohexyl formaldehyde are reacted in toluene to obtain the compound shown in formula P1, and the specific steps are:
[0068] In a 250ml three-necked flask, add 12.8 grams of 3,4-dihydroxy-1-vinylcyclopentane (prepared with reference to Tetrahedron60 (2004) 8113 8130), 23.6 grams of propyl biscyclohexyl formaldehyde, 100 milliliters of toluene, heated to 80 degrees React for 4 hours, cool down, dry over magnesium sulfate, separate by silica gel column chromatography, recrystallize the obtained cis-trans mixed substance with a mixed solvent of ethanol and petroleum ether to obtain a trans product, 9.5 grams of the compound shown in formula P1, and obtain Yield 27.5%, product mass spectrum (m / e): 334, 1 HNMR (500MHz, CDCl3) δ5.84(m, 1H), δ5.01(m, 1H), δ4.94(m,...
Embodiment 2
[0070] The concrete compound of synthesis is shown in formula P3:
[0071]
[0072] The synthetic route of P3 is as follows:
[0073]
[0074] The synthetic method refers to P1, but propyl biscyclohexyl formaldehyde is replaced with propyl phenyl cyclohexyl formaldehyde to obtain 6.9 grams of compound shown in formula P3, product mass spectrum (m / e): 340, 1 HNMR (500MHz, CDCl 3 )δ7.20~7.0(m, 4H), δ5.90(m, 1H), δ5.01(m, 1H), δ4.93(m, 1H), δ4.72(d, 1H), δ4. 35(m, 2H), δ2.61(t, 2H), δ2.60~2.38(m, 2H), δ2.02(m, 1H), δ1.96~1.55(m, 8H), δ1.41 (m, 2H), δ 1.20 (m, 2H), δ 1.03 (m, 2H), δ 0.94 (t, 3H).
Embodiment 3
[0076] The concrete compound of synthesis is shown in formula P2:
[0077]
[0078] The synthetic route of P2 is as follows:
[0079]
[0080] That is, the compound 4-propyl-1,2-cyclopentanediol and vinyl biscyclohexyl formaldehyde are reacted in toluene to obtain the compound shown in formula P2, and the specific steps are:
[0081] In a 250ml three-necked flask, add 14.4 grams of 4-propyl-1,2-cyclopentanediol (prepared with reference to European Journal of Medicinal Chemistry 46 (2011) 1263-1273), 22.0 grams of vinyl biscyclohexyl formaldehyde (prepared with reference to EP1860089) , 100 milliliters of toluene, heated to 80 degrees and reacted for 4 hours, lowered the temperature, dried over magnesium sulfate, separated by silica gel column chromatography, and recrystallized the obtained cis-trans mixed substance with ethanol and sherwood oil mixed solvent to obtain the trans product, such as 6.1 grams of compound shown in formula P2, yield 17.6%, product mass spectru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com