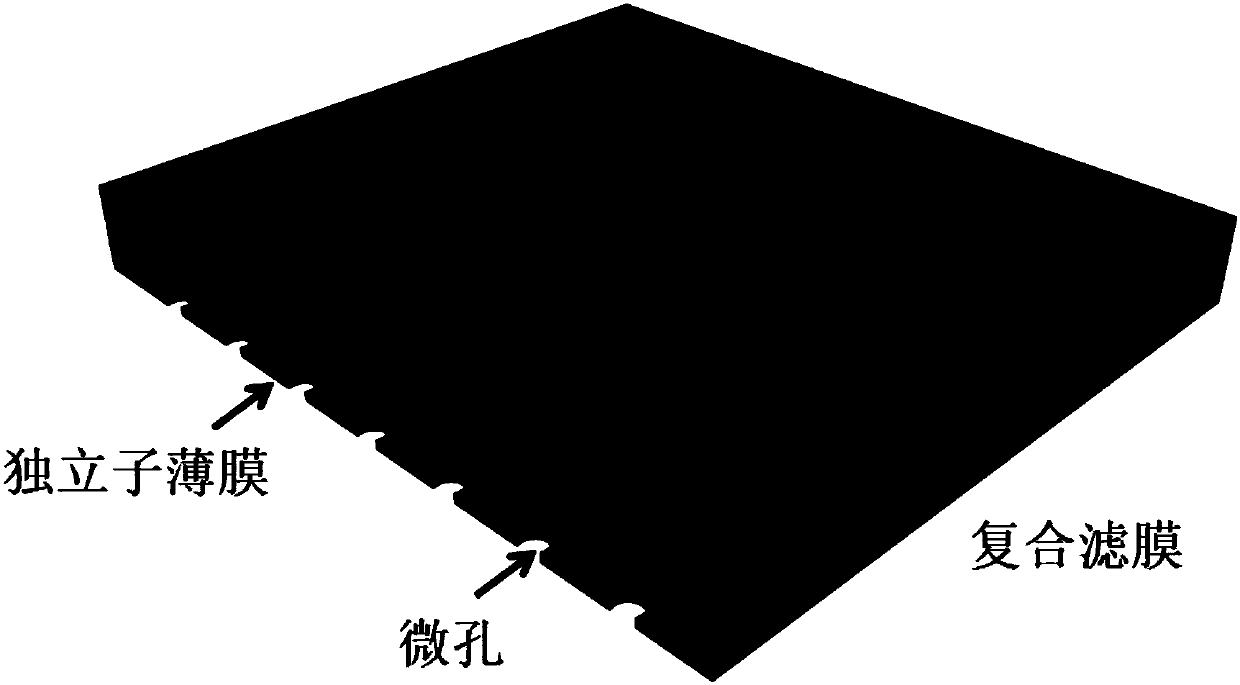
Rare cell unicellular level separating and detection method
A rare cell and single cell technology, which is applied in the field of rare cell separation and detection, can solve the problems of difficult single cell recovery, non-automation, and complicated enrichment process, so as to avoid the loss rate of rare cells, save time and cost, and save The effect of the enrichment step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] (1) Sample preparation:
[0087] 100 non-small cell lung cancer cells H1975 were stained with 3 ul of the dye VybrantDil (Life Tehnologies) for 1 hour. The tumor cells marked by this staining can be identified by fluorescence on the composite filter membrane after filtration. After staining, the cells were spiked into 2ml of blood.
[0088] (2) Pretreatment before filtration (removal of red blood cells)
[0089] The lysed red blood cells were treated with reference to the method in Test Example 1, and similarly, the mixture of H1975 cells and white blood cells was suspended in 1 ml of HBSS, and the cells were counted.
[0090] (3) filter
[0091] Refer to the filtration method of Test Example 9, that is, the filtration pressure is 20mba, the concentration of the sampled cell solution is 500,000 cells / ml, and the total sampled volume is 4 million cells.
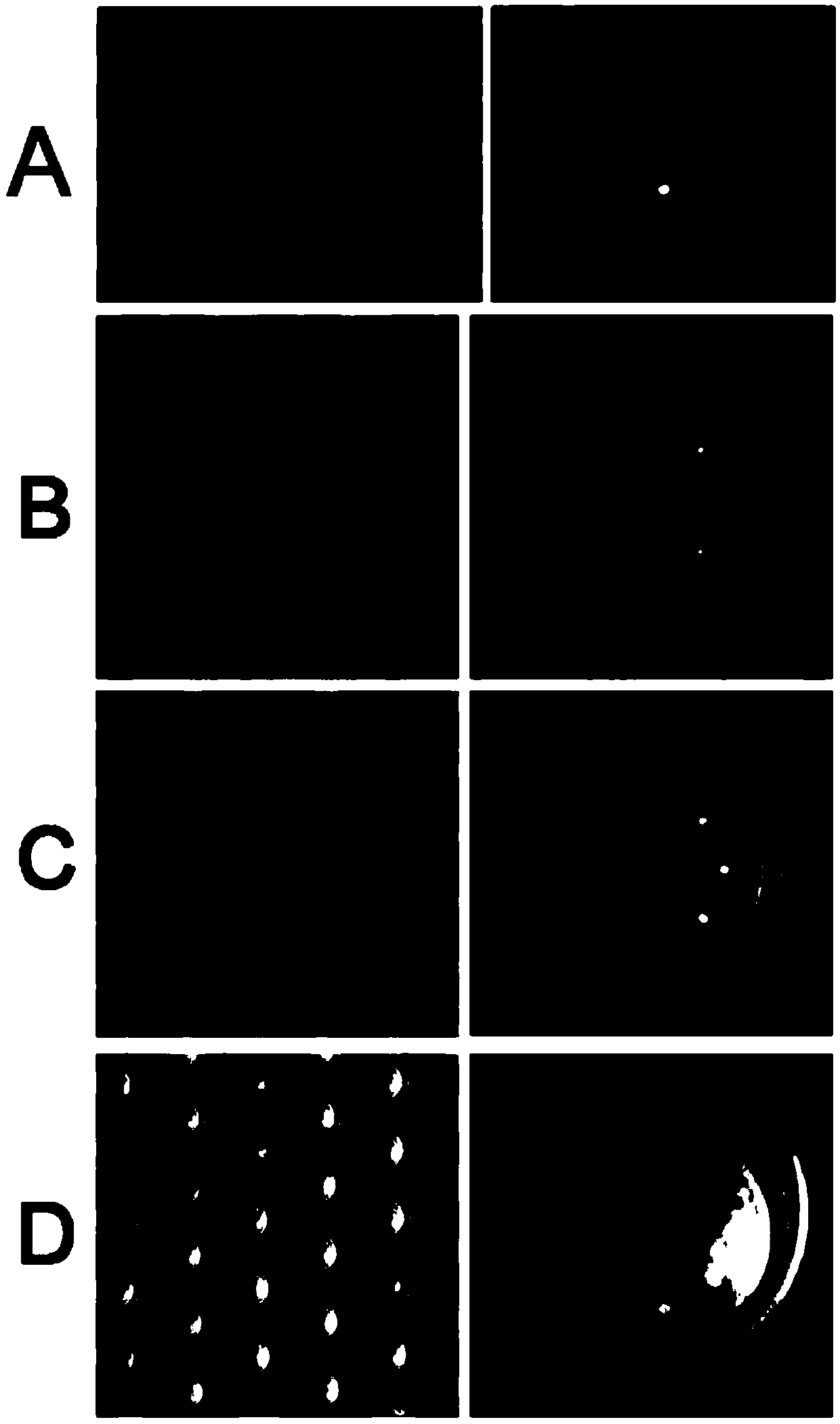
[0092] After the filtration, the composite filter membrane was scanned for fluorescence, and the number of positi...
Embodiment 2
[0133] Objective: To prove the feasibility of the method of the present invention in the isolation of lung adenocarcinoma cells.
[0134] according to Figure 8 The process is to isolate and identify circulating tumor cells from peripheral blood of patients, and the specific steps are:
[0135] 1. Take 3ml of blood sample of lung adenocarcinoma known to have the L858R mutation in situ, and lyse the red blood cells (the same step as the red blood cell lysing in the test (1))
[0136] 2. Add 1ml of buffer to the cell pellet in the centrifuge tube, mix by pipetting and transfer to a 1.5ml centrifuge tube.
[0137] 3. Cell counting, calculate the total amount of cells in the cell suspension (about 4 million).
[0138] 4. Add cell staining reagents according to the number of cells (add 10ul CD45 and 10ulEpCAM to 5 million white blood cells, increase the amount of antibodies proportionally if the number of cells is greater than 5 million, and add antibodies to the amount of 500 ce...
Embodiment 3
[0150] Objective: To prove the feasibility of the method of the present invention in gastric cancer.
[0151] The blood sample was taken from a gastric cancer patient, and the steps 1-12 were the same as those in Example 2. After amplification, use Sanger to detect gene mutations. (eg EGFR, KRAS, TP53, etc.), A750T mutation was detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com