Clinical test item data processing method and device and electronic device
A technology for detecting items and data, applied in the field of biomedicine, can solve the problems of deviation of analysis results, EHR data inclusion, uneven distribution of data, etc., to achieve the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
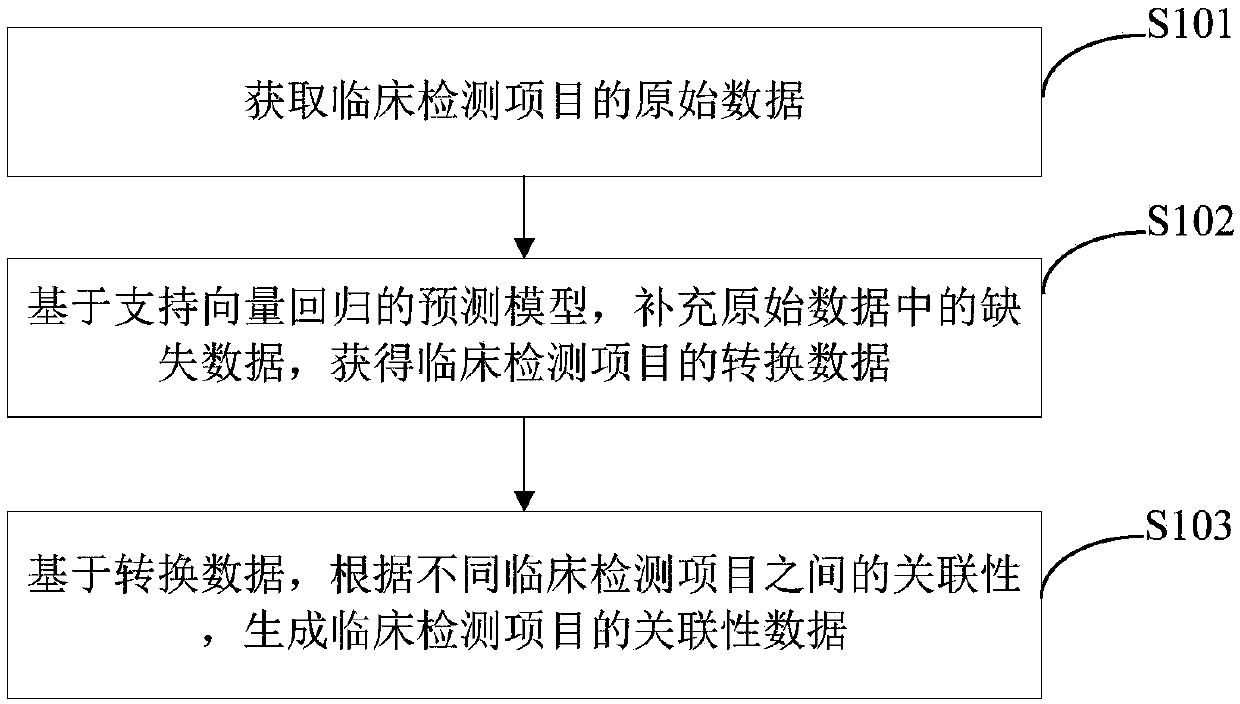
[0048] Such as figure 1 As shown, the embodiment of the present invention provides a data processing method for clinical testing items, which can be applied to the field of electronic health records (EHR) big data, and the method includes:
[0049] Step S101: Acquiring raw data of clinical testing items.
[0050] Here, the clinical testing items are defined as "clinotype", such as objectively measured neutrophil percentage, heart rate and 2h postprandial blood glucose and other clinical information. The following is a brief description of it:
[0051] Clinical testing items (clinotype) do not include testing items related to the two types of treatment that can use biomedical equipment for treatment and diagnosis. Most clinotypes are hospital testing items. It should be noted that the clinotype is not exactly the same as the hospital testing items because of the following reasons: firstly, with the development of modern mobile phone electronic devices, patients can perform the...
Embodiment 2
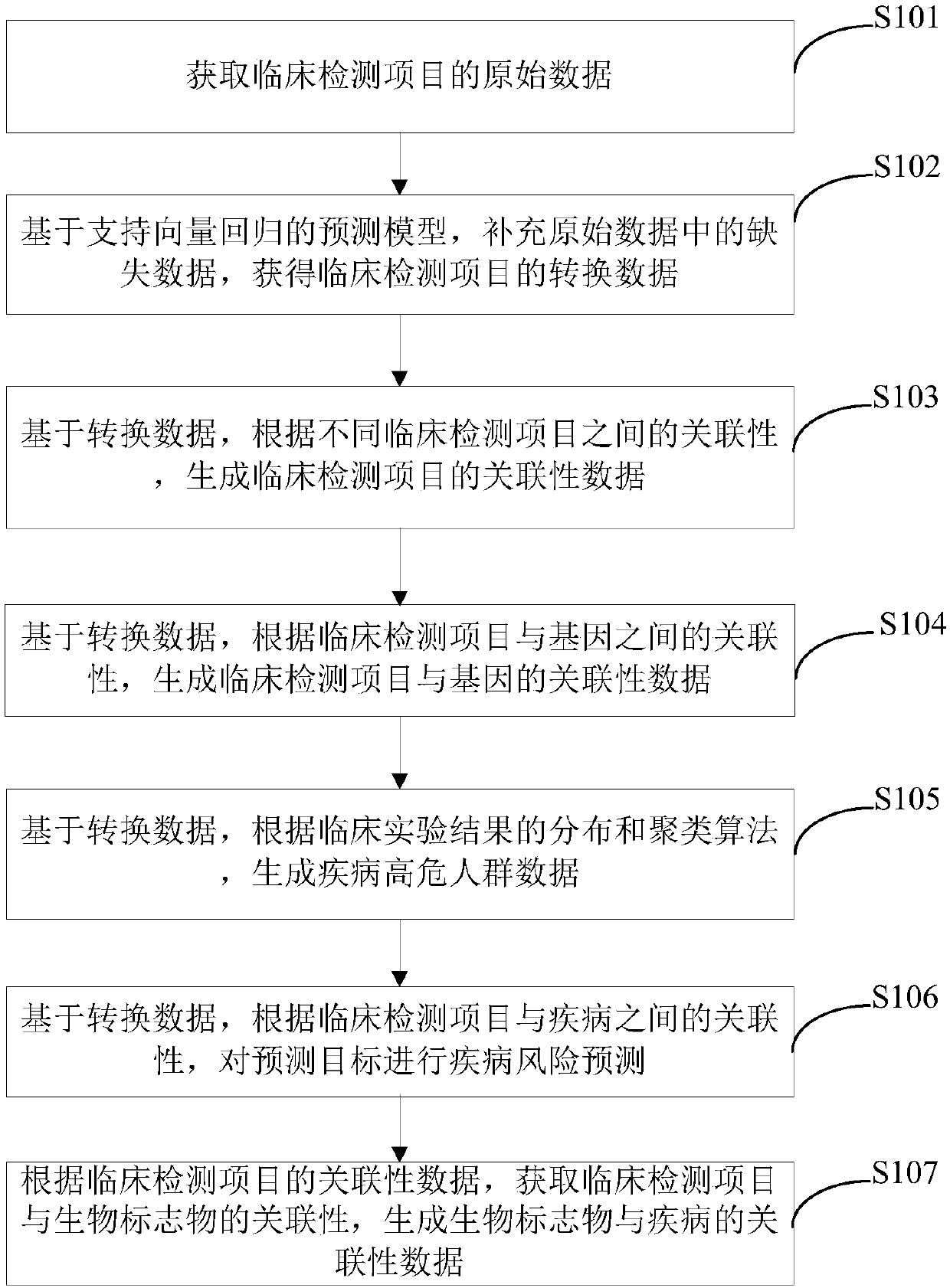
[0083] Such as image 3 As shown, the embodiment of the present invention provides another data processing method of clinical testing items, which can be applied to the field of electronic health records (EHR) big data, and the method includes:
[0084] Step S101: Acquiring raw data of clinical testing items.
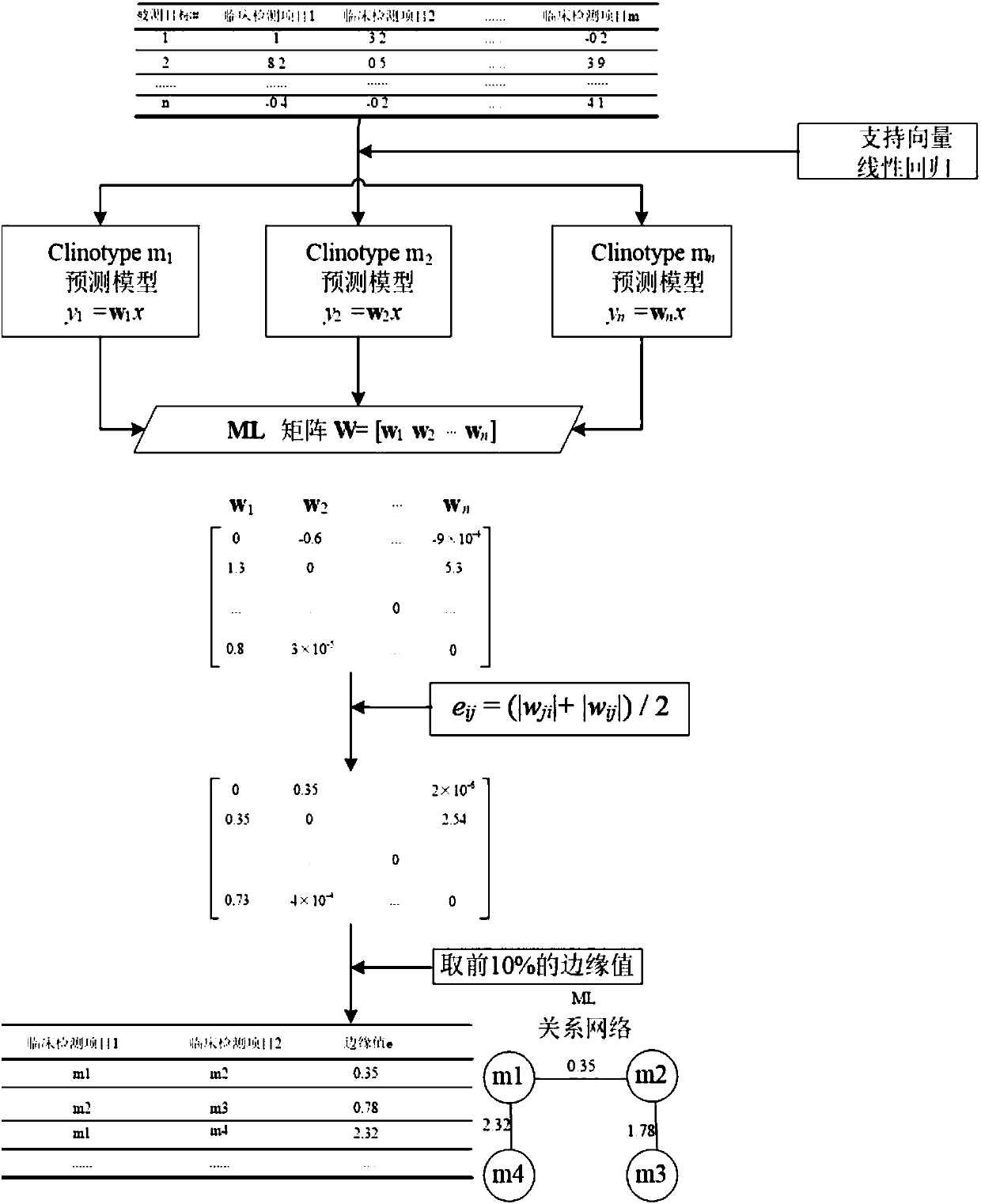
[0085] Step S102: Based on the prediction model of support vector regression, the missing data in the original data is supplemented to obtain the converted data of the clinical testing items.
[0086] Step S103: Based on the conversion data, according to the correlation between different clinical testing items, generate the correlation data of the clinical testing items.
[0087] Step S104: Based on the conversion data, according to the correlation between the clinical testing items and the genes, generate the correlation data between the clinical testing items and the genes.
[0088]The correlation data between the above-mentioned clinical detection items and genes c...
Embodiment 3
[0115] see Figure 6 , the embodiment of the present invention also provides a data processing device for clinical testing items, including:
[0116] Obtaining module 10, for obtaining the raw data of clinical examination item;
[0117] Conversion module 20, for the predictive model based on support vector regression, supplements the missing data in the original data, obtains the conversion data of clinical detection item;
[0118] Association module 30, is used for based on conversion data, according to the association between different clinical examination items, generates the correlation data of clinical examination item.
[0119] Further, the correlation module 30 is also used for generating correlation data between clinical testing items and genes based on the conversion data and according to the correlation between clinical testing items and genes.
[0120] Preferably, the association module 30 is also used to generate disease high-risk population data based on the con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com