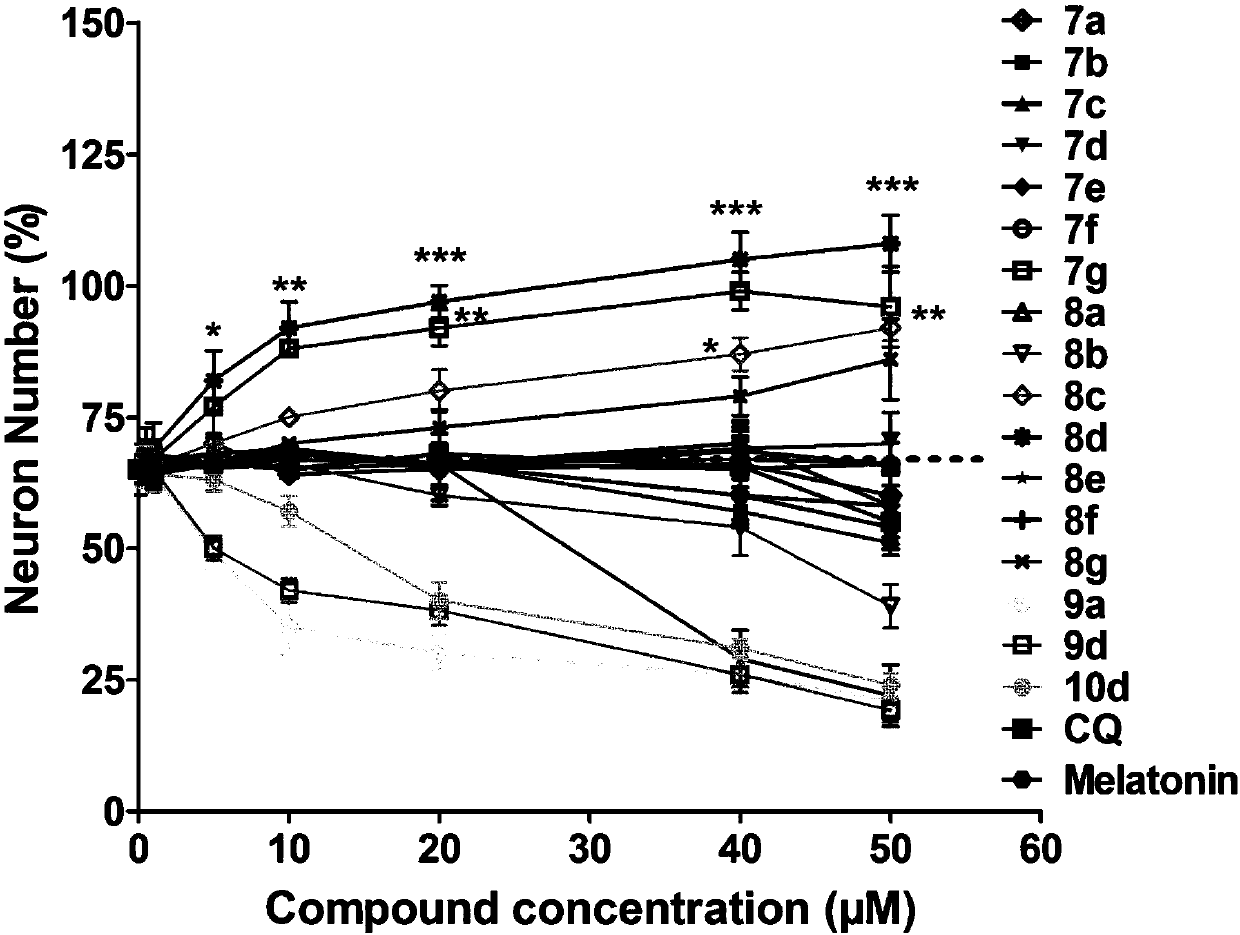
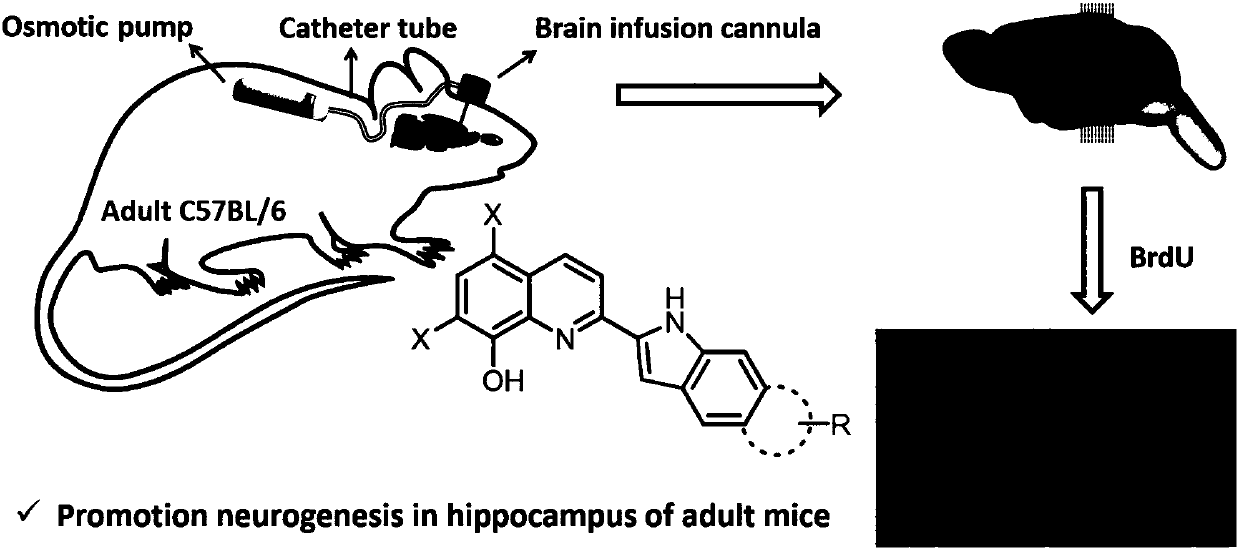
Quinoline-indole derivative and application thereof in preparation of drug used for treating alzheimer disease
A technology of Alzheimer's disease and indole derivatives, applied in the field of medicine, can solve the problems of irreparable neuron cells, achieve the number of induced neurons, improve cognitive ability and spatial memory, improve spatial memory and cognition effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: 2-(5-Hydroxy-1-H-indole-2-substituted)-8-hydroxyquinoline
[0038]
[0039] (1) Step 1: Synthesis of (E)-2-(5-acetoxy-2-nitro)-8-acetoxyquinoline
[0040]
[0041]Add 1.59g (10mmol) 2-methyl-8-hydroxyquinoline and 1.67g (10mmol) 2-nitro-5-hydroxybenzaldehyde to a 100mL round bottom bottle, 16mL acetic anhydride, stir at 150°C for 12h, After cooling to room temperature, add 50 mL of water, stir at room temperature for 1 hour, filter with suction, wash with water, and dry in vacuo to obtain a yellow solid (E)-2-(5-Acetoxy-2-nitro)-8-Acetoxy Quinoline 3.12g, yield 81%. R f =0.36 (petroleum / EtOAc=2 / 1). 1 H NMR (400MHz, CDCl 3 )δ8.27(d, J=16.0Hz, 1H), 8.16(d, J=8.5Hz, 1H), 8.08(d, J=8.9Hz, 1H), 7.68(dd, J=8.0, 1.1Hz, 1H), 7.62(d, J=8.6Hz, 1H), 7.60(d, J=2.4Hz, 1H), 7.50(t, J=7.8Hz, 1H), 7.44(dd, J=7.4, 1.2Hz, 1H), 7.28(d, J=16.0Hz, 1H), 7.22(dd, J=8.9, 2.4Hz, 1H), 2.57(s, 3H), 2.37(s, 3H).LC / MS(ESI): 393.1[M+H] + .
[0042] (2) Step 2: Synthesis of ...
Embodiment 2
[0048] Example 2: 2-(1-H-indole-2-substituted)-8-hydroxyquinoline
[0049]
[0050] The specific implementation steps are similar to Example 1, add 2.9g 2-(1-H-indole-2-substituted)-8-acetoxyquinoline and 10mL anhydrous methanol to a 100mL round bottom bottle, add 1.0 times Equivalent K 2 CO 3 . Stir at room temperature for 1 h, add 60 mL of water after the reaction is complete, filter with suction, separate by silica gel column chromatography, and elute with ethyl acetate to obtain a light yellow oily substance, namely 2-(1-H-indole-2-substituted)-8-hydroxy quinoline. Concrete implementation steps are similar to Example 1, and the productive rate is 83%. R f =0.35 (petroleum / EtOAc=1 / 1), mp 241.5-242.3°C. 1 H NMR (400MHz, CDCl 3 )δ9.54(s,1H),8.13(d,J=8.7Hz,1H),7.91(d,J=8.6Hz,1H),7.69(d,J=8.0Hz,1H),7.47(dd, J=8.2,0.8Hz,1H),7.43–7.36(m,1H),7.34–7.25(m,2H),7.22–7.16(m,2H),7.16–7.09(m,1H). 13 C NMR (101MHz, CDCl 3 )δ151.76,148.17,137.48,137.06,136.73,136.23,128.99,127...
Embodiment 3
[0051] Example 3: 2-(5-fluoro-1-H-indole-2-substituted)-8-hydroxyquinoline
[0052]
[0053] The specific implementation steps are similar to Example 1, add 2.9g 2-(5-fluoro-1-H-indole-2-substituted)-8-acetoxyquinoline and 10mL anhydrous methanol in the round bottom bottle of 100mL , add 1.0 times the equivalent of K 2 CO 3 . Stir at room temperature for 1 h, add 60 mL of water after the reaction is complete, filter with suction, separate by silica gel column chromatography, and elute with ethyl acetate to obtain a light yellow oily substance, namely 2-(5-fluoro-1-H-indole-2-substituted) -8-Hydroxyquinoline. Yield 91%. R f =0.39 (petroleum / EtOAc=1 / 1), mp 257.2-257.8°C. 1 HNMR (400MHz, DMSO) δ8.36 (d, J = 8.6Hz, 1H), 8.13 (d, J = 8.6Hz, 1H), 7.53 (dd, J = 8.7, 4.4Hz, 1H), 7.49–7.38 ( m,3H),7.36(s,1H),7.16(d,J=7.1Hz,1H),7.10(td,J=9.3,2.5Hz,1H). 13 C NMR (101MHz, DMSO) δ158.80(s), 156.49(s), 153.47(s), 147.83(s), 139.31(s), 137.76(s), 137.32(s), 134.24(s), 129.19 (d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com