Application of amantadine-gardenia amide A heterozygote compound in drug for treating Parkinson's disease
A technology of gardeniamide and amantadine, which is applied in the field of medicine, can solve problems such as drug side effects, and achieve high safety, safe and effective neuroprotective effect, and strong neuroprotective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
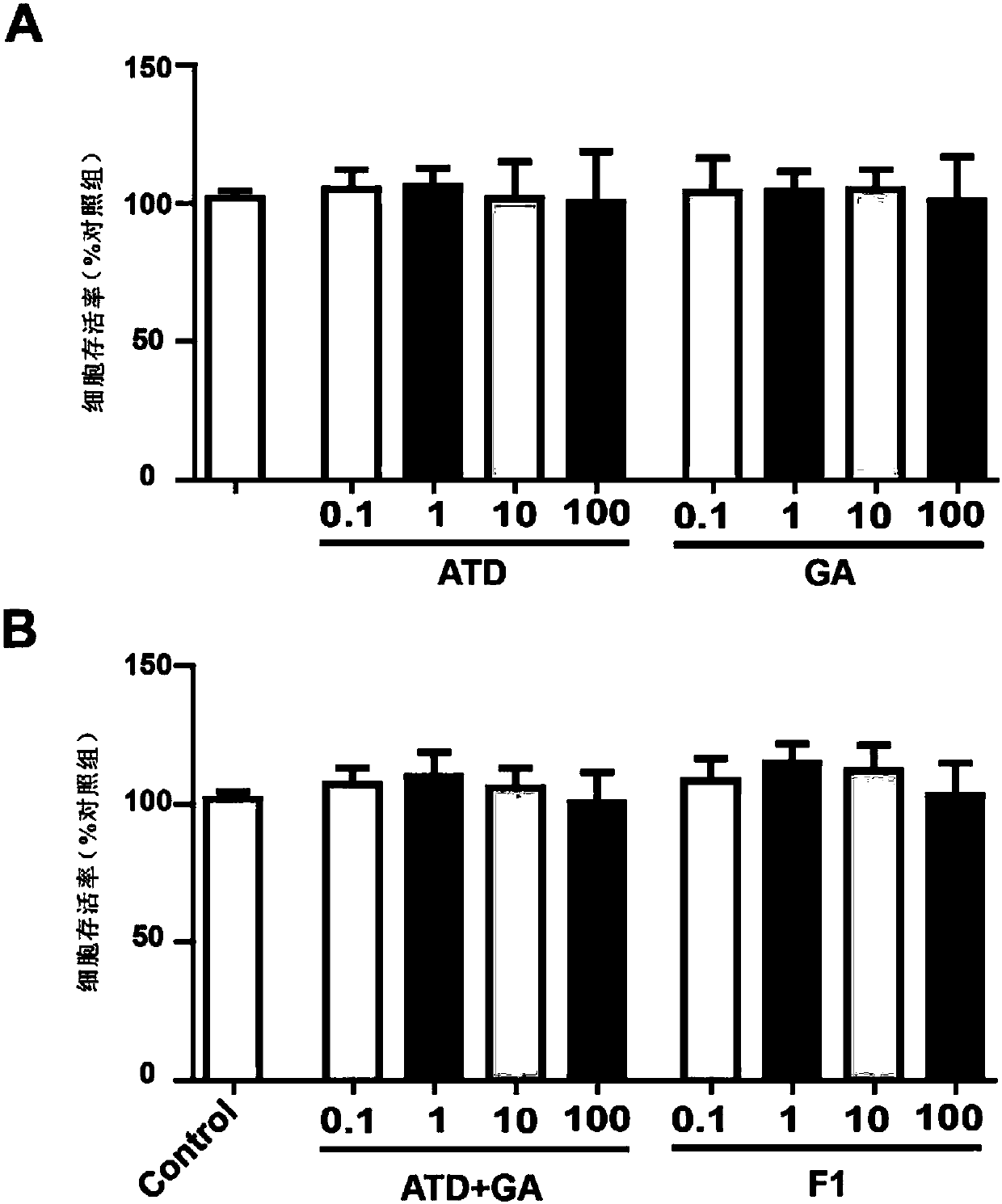
[0036] Cytotoxicity of compound F1 against normal SH-SY5Y.
[0037] Experimental material: human neuroblastoma cell line SH-SY5Y, purchased from the Cell Bank of the Chinese Academy of Sciences in Shanghai
[0038] Experimental procedure: Digest SH-SY5Y cells in the logarithmic phase, centrifuge, resuspend the cells in complete medium containing 10% fetal bovine serum FBS, and inoculate 5×10 3 The SH-SY5Y cells per well were placed in a 96-well plate, and the volume of the cell suspension in each well was 100 μL. Four concentrations of 10 and 100 μM, each with 8 replicate wells, were cultured in a constant temperature incubator for 24 hours. After adding different concentrations of medicinal solutions for pretreatment for 3 hours, discard the old medicinal solutions, wash once with PBS, add 25 μL of MTT with a concentration of 5 mg / mL to each well, and after incubation for 3 hours, absorb the MTT solution, add 150 μL of fresh DMSO to each well, Shake for 10 min, and use an e...
Embodiment 2
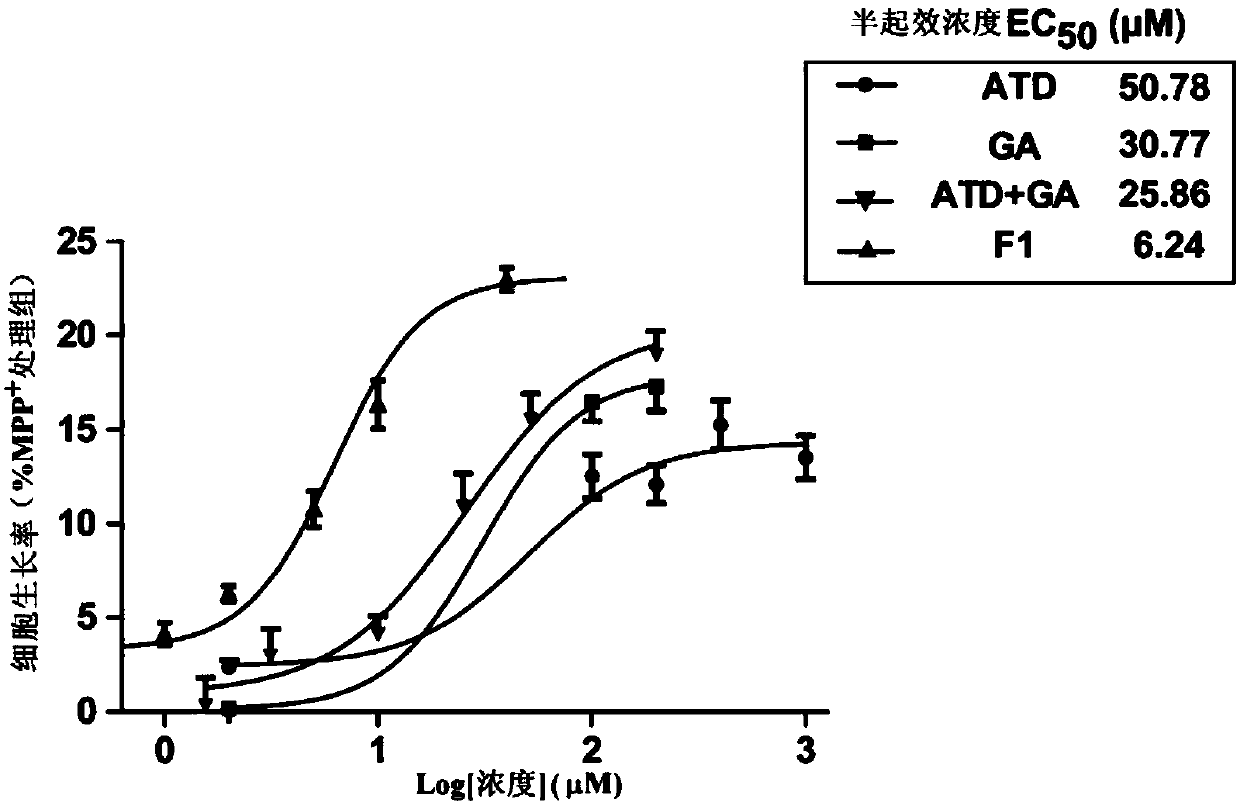
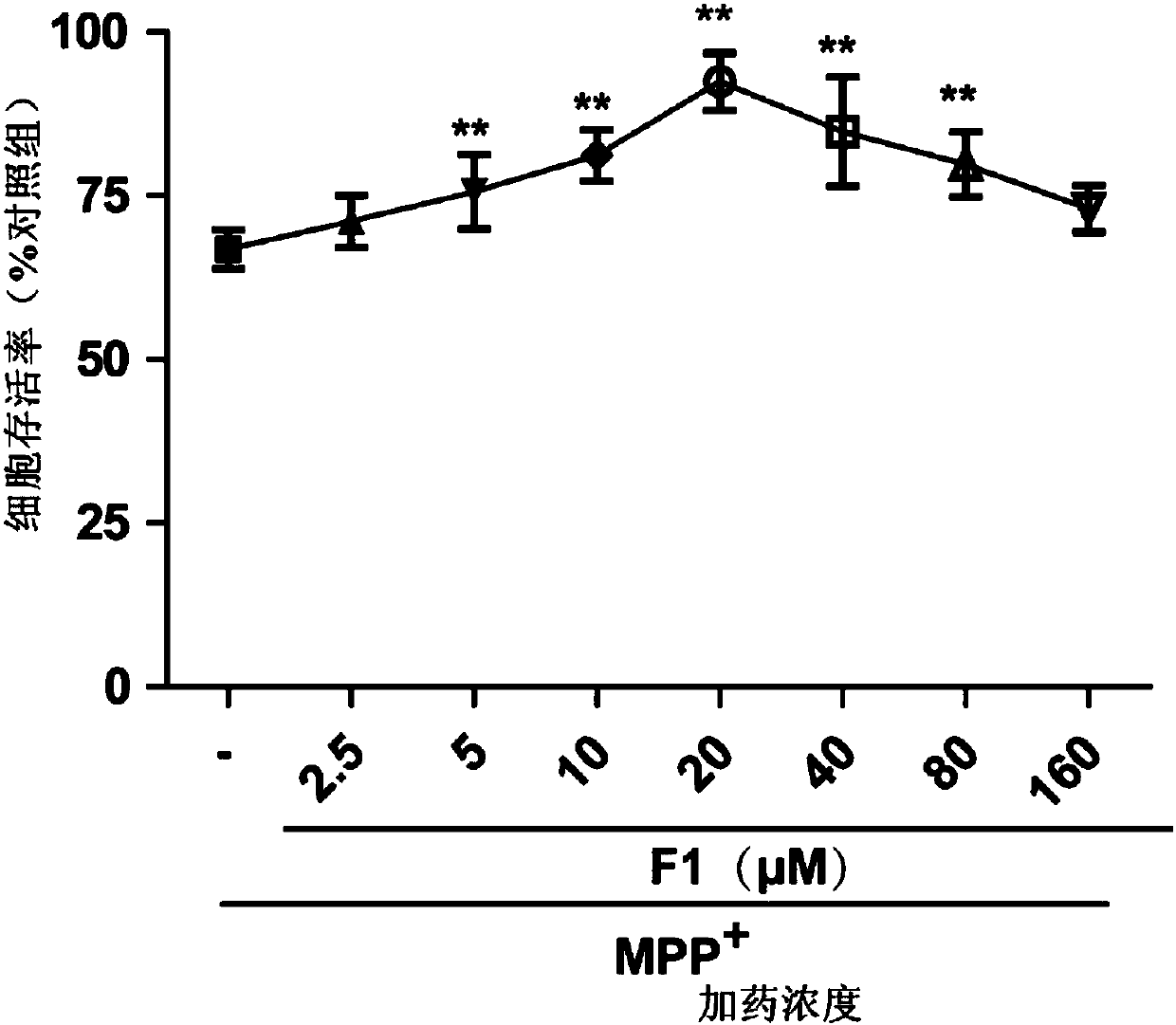
[0042] Detection of F1 to MPP by CCK8 method + Influence of damaged SH-SY5Y cell viability.
[0043] Experimental procedure: Set blank group, control group, ATD group, GA group, ATD+GA group and F1 group in a 96-well plate, and set seven concentrations of 2.5, 5, 10, 20, 40, 80 and 160 μM in each group, and add 2mM modeling drug MPP + Processing 24h. Add 10 μL of CCK8 solution directly to each well, and finally place it in a microplate reader for measurement at a wavelength of 450 nm, and calculate the inhibition rate.
[0044] According to the experimental results, take the logarithm value of the sample concentration as the abscissa, and the inhibition rate as the ordinate, draw the inhibition curve, and calculate the half-maximum effect concentration EC of different samples according to the curve 50 . See the experimental results figure 2 , indicating that F1 and its prodrugs have an effect on MPP + Injured SH-SY5Y cells have a certain degree of neuroprotection, EC 5...
Embodiment 3
[0047] Examining F1 vs. MPP + Effects on morphology of injured SH-SY5Y cells.
[0048] According to the steps in Example 2, the normal cells and the drug-treated cells were observed under the same microscope and the same lens, and the morphology of the cells was recorded.
[0049] See the experimental results Figure 4 . The results showed that normal SH-SY5Y cells were larger in volume, smaller in gaps, and full-bodied in polysynaptic shape. When it is subject to MPP + After injury, the number of cells decreased and the morphology was long spindle. When pre-administered 20 μM F1 to protect cells, compared with the model group, the cell morphology and cell density were significantly improved; while the cell morphology of the ATD group had no significant change, and both the GA and ATD+GA groups had a certain degree of protection. The results showed that the neuroprotective effect of F1 was better than that of its prodrug and its combined drugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com