Novel method for preparing chromanol derivative
A compound and mixture technology, applied in the field of preparation of chiral active chromanol derivatives, can solve the problems of high production cost, inapplicability to large-scale production, low yield, etc., achieve excellent yield, and benefit large-scale The effect of large-scale production and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
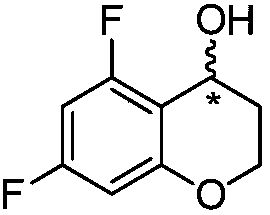
[0062] Example 1: Preparation of (R)-5,7-difluorochroman-4-ol
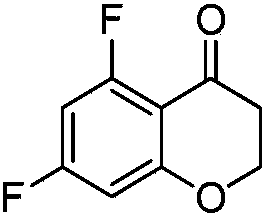
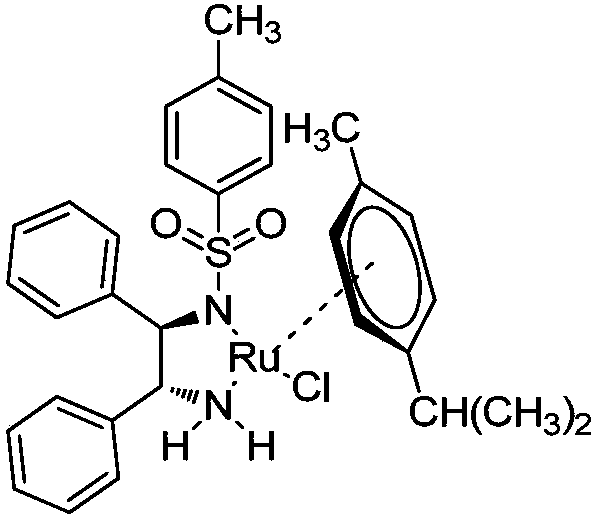
[0063] Put 30g of triethylamine into the reactor and cool to -10°C. 27 g of formic acid were added slowly at a temperature below 10°C. 56 mg of ruthenium catalyst RuCl(p-cymene)[(R,R)-Ts-DPEN] was added to the reactor. 33 g of 5,7-difluorochroman-4-one dissolved in 87 g of tetrahydrofuran was added to the reactor at a temperature below 10°C. The reaction temperature was raised to 40°C. After the reaction was completed, it was cooled to room temperature, 293 g of ethyl acetate and 163 g of pure water were added with stirring, and the organic layer was separated. Concentrate under reduced pressure at a temperature lower than 40°C, add 222 g of heptane, stir at 25°C, and filter the resulting solid. It was then dried under vacuum at 40°C to afford (R)-5,7-difluorochroman-4-ol (30 g, 91% and 100% ee).
[0064] 1 H-NMR (270MHz, CDCl 3 )δ:6.47-6.36(m,2H),5.05-4.97(m,1H),4.36-4.20(m,2H),2.16-1.92(m,3H)ppm
[0065]...
Embodiment 2
[0066] Example 2: Preparation of (S)-5,7-difluorochroman-4-ol
[0067] Put 30g of triethylamine into the reactor and cool to -10°C. 27 g of formic acid were added slowly at a temperature below 10°C. 56 mg of ruthenium catalyst RuCl(p-cymene)[(S,S)-Ts-DPEN] was added to the reactor. 33 g of 5,7-difluorochroman-4-one dissolved in 87 g of tetrahydrofuran was added to the reactor at a temperature below 10°C. The reaction temperature was raised to 40°C. After the reaction was completed, it was cooled to room temperature, 293 g of ethyl acetate and 163 g of pure water were added with stirring, and the organic layer was separated. Concentrate under reduced pressure at a temperature lower than 40°C, add 222 g of heptane, stir at 25°C, and filter the resulting solid. It was then dried under vacuum at 40°C to give (S)-5,7-difluorochroman-4-ol (28 g, 85% and 100% ee).
[0068] 1 H-NMR: Spectrum data are the same as those of (R)-5,7-difluorochroman-4-ol (ie Example 1).
[0069] Chi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com